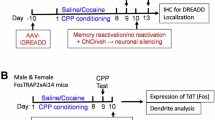
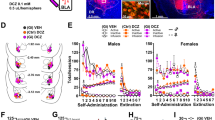
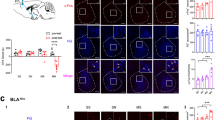
Abstract
Drug-associated conditioned cues promote subjects to recall drug reward memory, resulting in drug-seeking and reinstatement. A consolidated memory becomes unstable after recall, such that the amnestic agent can disrupt the memory during the reconsolidation stage, which implicates a potential therapeutic strategy for weakening maladaptive memories. The basolateral amygdala (BLA) involves the association of conditioned cues with reward and aversive valences and projects the information to the nucleus accumbens (NAc) that mediates reward-seeking. However, whether the BLA-NAc projection plays a role in drug-associated memory reactivation and reconsolidation is unknown. We used methamphetamine (MeAM) conditioned place preference (CPP) to investigate the role of BLA-NAc neural projection in the memory reconsolidation. Two weeks before CPP training, we infused adeno-associated virus (AAV) carrying the designer receptor exclusively activated by designer drugs (DREADD) or control constructs. We infused clozapine-N-oxide (CNO) after the recall test to manipulate the neural activity of BLA-NAc projections in mice. We found that after recall, DREADD-mediated inhibition of BLA neurons projecting to the NAc core blunted consolidated MeAM-associated memory. Inhibition of BLA glutamatergic nerve terminals in the NAc core 1 h after recall disrupted consolidated MeAM-associated memory. However, inhibiting this pathway after the time window of reconsolidation failed to affect memory. Furthermore, under the condition without memory retrieval, DREADD-mediated activation of BLA-NAc core projection was required for amnesic agents to disrupt consolidated MeAM-associated memory. Our findings provide evidence that the BLA-NAc pathway activity is involved in the post-retrieval processing of MeAM-associated memory in CPP.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–9. https://doi.org/10.1038/nn1579
Gipson CD, Kupchik YM, Shen H, Reissner KJ, Thomas CA, Kalivas PW. Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron. 2013;77:867–72. https://doi.org/10.1016/j.neuron.2013.01.005
Sampedro-Piquero P, Ladrón de Guevara-Miranda D, Pavón FJ, Serrano A, Suárez J, Rodríguez de Fonseca F, et al. Neuroplastic and cognitive impairment in substance use disorders: a therapeutic potential of cognitive stimulation. Neurosci Biobehav Rev. 2019;106:23–48. https://doi.org/10.1016/j.neubiorev.2018.11.015
Bossert JM, Liu SY, Lu L, Shaham Y. A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci. 2004;24:10726–30. https://doi.org/10.1523/jneurosci.3207-04.2004
Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27:12655–63. https://doi.org/10.1523/jneurosci.3926-07.2007
LeBlanc KH, Ostlund SB, Maidment NT. Pavlovian-to-instrumental transfer in cocaine seeking rats. Behav Neurosci. 2012;126:681–9. https://doi.org/10.1037/a0029534
Nader K. Memory traces unbound. Trends Neurosci. 2003;26:65–72. https://doi.org/10.1016/s0166-2236(02)00042-5
Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–6. https://doi.org/10.1038/35021052
Exton-McGuinness MTJ, Milton AL. Reconsolidation blockade for the treatment of addiction: challenges, new targets, and opportunities. Learn Mem. 2018;25:492–500. https://doi.org/10.1101/lm.046771.117
Lee JLC, Nader K, Schiller D. An update on memory reconsolidation updating. Trends Cogn Sci. 2017;21:531–45. https://doi.org/10.1016/j.tics.2017.04.006
Milton AL, Everitt BJ. The persistence of maladaptive memory: addiction, drug memories and anti-relapse treatments. Neurosci Biobehav Rev. 2012;36:1119–39. https://doi.org/10.1016/j.neubiorev.2012.01.002
Ben Mamou C, Gamache K, Nader K. NMDA receptors are critical for unleashing consolidated auditory fear memories. Nat Neurosci. 2006;9:1237–9. https://doi.org/10.1038/nn1778
Yu YJ, Huang CH, Chang CH, Gean PW. Involvement of protein phosphatases in the destabilization of methamphetamine-associated contextual memory. Learn Mem. 2016;23:486–93. https://doi.org/10.1101/lm.039941.115
Ambroggi F, Ishikawa A, Fields HL, Nicola SM. Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron. 2008;59:648–61. https://doi.org/10.1016/j.neuron.2008.07.004
Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015;517:284–92. https://doi.org/10.1038/nature14188
Keefer SE, Gyawali U, Calu DJ. Choose your path: divergent basolateral amygdala efferents differentially mediate incentive motivation, flexibility and decision-making. Behav Brain Res. 2021;409:113306 https://doi.org/10.1016/j.bbr.2021.113306
Koob GF. A basolateral amygdala microcircuit for drug craving: is there a craving engram. Biol Psychiatry. 2021;89:323–5. https://doi.org/10.1016/j.biopsych.2020.11.008
Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, et al. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–86. https://doi.org/10.1016/s0893-133x(01)00371-2
Chen YY, Zhang LB, Li Y, Meng SQ, Gong YM, Lu L, et al. Post-retrieval extinction prevents reconsolidation of methamphetamine memory traces and subsequent reinstatement of methamphetamine seeking. Front Mol Neurosci. 2019;12:157 https://doi.org/10.3389/fnmol.2019.00157
Shiflett MW, Balleine BW. At the limbic-motor interface: disconnection of basolateral amygdala from nucleus accumbens core and shell reveals dissociable components of incentive motivation. Eur J Neurosci. 2010;32:1735–43. https://doi.org/10.1111/j.1460-9568.2010.07439.x
Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–80. https://doi.org/10.1038/nature10194
Di Ciano P, Everitt BJ. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J Neurosci. 2004;24:7167–73. https://doi.org/10.1523/JNEUROSCI.1581-04.2004
Scofield MD, Heinsbroek JA, Gipson CD, Kupchik YM, Spencer S, Smith AC, et al. The nucleus accumbens: mechanisms of addiction across drug classes reflect the importance of glutamate homeostasis. Pharm Rev. 2016;68:816–71. https://doi.org/10.1124/pr.116.012484
Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7:389–97. https://doi.org/10.1038/nn1217
Rossi LM, Reverte I, Ragozzino D, Badiani A, Venniro M, Caprioli D. Role of nucleus accumbens core but not shell in incubation of methamphetamine craving after voluntary abstinence. Neuropsychopharmacology. 2020;45:256–65. https://doi.org/10.1038/s41386-019-0479-4
Puaud M, Higuera-Matas A, Brunault P, Everitt BJ, Belin D. The basolateral amygdala to nucleus accumbens core circuit mediates the conditioned reinforcing effects of cocaine-paired cues on cocaine seeking. Biol Psychiatry. 2021;89:356–65. https://doi.org/10.1016/j.biopsych.2020.07.022
Kim JH, Jung AH, Jeong D, Choi I, Kim K, Shin S, et al. Selectivity of neuromodulatory projections from the basal forebrain and locus ceruleus to primary sensory cortices. J Neurosci. 2016;36:5314–27. https://doi.org/10.1523/JNEUROSCI.4333-15.2016
Li SJ, Vaughan A, Sturgill JF, Kepecs A. A viral receptor complementation strategy to overcome CAV-2 tropism for efficient retrograde targeting of neurons. Neuron. 2018;98:905–17.e5. https://doi.org/10.1016/j.neuron.2018.05.028
Namburi P, Beyeler A, Yorozu S, Calhoon GG, Halbert SA, Wichmann R, et al. A circuit mechanism for differentiating positive and negative associations. Nature. 2015;520:675–8. https://doi.org/10.1038/nature14366
Morris RG, Inglis J, Ainge JA, Olverman HJ, Tulloch J, Dudai Y, et al. Memory reconsolidation: sensitivity of spatial memory to inhibition of protein synthesis in dorsal hippocampus during encoding and retrieval. Neuron. 2006;50:479–89. https://doi.org/10.1016/j.neuron.2006.04.012
Nader K, Hardt O. A single standard for memory: the case for reconsolidation. Nat Rev Neurosci. 2009;10:224–34. https://doi.org/10.1038/nrn2590
Deroche MA, Lassalle O, Castell L, Valjent E, Manzoni OJ. Cell-type- and endocannabinoid-specific synapse connectivity in the adult nucleus accumbens core. J Neurosci. 2020;40:1028–41. https://doi.org/10.1523/JNEUROSCI.1100-19.2019
Medina JH, Viola H. ERK1/2: a key cellular component for the formation, retrieval, reconsolidation and persistence of memory. Front Mol Neurosci. 2018;11:361 https://doi.org/10.3389/fnmol.2018.00361
Wells AM, Arguello AA, Xie X, Blanton MA, Lasseter HC, Reittinger AM, et al. Extracellular signal-regulated kinase in the basolateral amygdala, but not the nucleus accumbens core, is critical for context-response-cocaine memory reconsolidation in rats. Neuropsychopharmacology. 2013;38:753–62. https://doi.org/10.1038/npp.2012.238
Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47:873–84. https://doi.org/10.1016/j.neuron.2005.08.006
Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward. Psychopharmacology. 2000;153:31–43. https://doi.org/10.1007/s002130000569
Visser E, Matos MR, Mitrić MM, Kramvis I, van der Loo RJ, Mansvelder HD, et al. Extinction of cocaine memory depends on a feed-forward inhibition circuit within the medial prefrontal cortex. Biol Psychiatry. 2021. https://doi.org/10.1016/j.biopsych.2021.08.008
Zhou Y, Zhu H, Liu Z, Chen X, Su X, Ma C, et al. A ventral CA1 to nucleus accumbens core engram circuit mediates conditioned place preference for cocaine. Nat Neurosci. 2019;22:1986–99. https://doi.org/10.1038/s41593-019-0524-y
Augur IF, Wyckoff AR, Aston-Jones G, Kalivas PW, Peters J. Chemogenetic activation of an extinction neural circuit reduces cue-induced reinstatement of cocaine seeking. J Neurosci. 2016;36:10174–80. https://doi.org/10.1523/jneurosci.0773-16.2016
Stefanik MT, Kupchik YM, Kalivas PW. Optogenetic inhibition of cortical afferents in the nucleus accumbens simultaneously prevents cue-induced transient synaptic potentiation and cocaine-seeking behavior. Brain Struct Funct. 2016;221:1681–9. https://doi.org/10.1007/s00429-015-0997-8
Stefanik MT, Kalivas PW. Optogenetic dissection of basolateral amygdala projections during cue-induced reinstatement of cocaine seeking. Front Behav Neurosci. 2013;7:213 https://doi.org/10.3389/fnbeh.2013.00213
Schroyens N, Beckers T, Kindt M. In search for boundary conditions of reconsolidation: a failure of fear memory interference. Front Behav Neurosci. 2017;11:65 https://doi.org/10.3389/fnbeh.2017.00065
Ma YY, Lee BR, Wang X, Guo C, Liu L, Cui R, et al. Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron. 2014;83:1453–67. https://doi.org/10.1016/j.neuron.2014.08.023
Acknowledgements
We thank Dr. Bryan Roth for gifts of chemogenetic constructs, and thank the Laboratory Animal Center, College of Medicine, National Cheng Kung University and Taiwan Animal Consortium for the technical support. We thank Dr. Li-Hsien Chen and the Bioimaging Core Facility of the National Core Facility for Biopharmaceuticals, Ministry of Science and Technology, Taiwan, for confocal technical support.
Funding
This study was supported by grants NHRI-EX109-10730NI from the National Health Research Institute and MOST110-2811-B-006-545, MOST109-2811-B-006-535, MOST110-2320-B-006-047, MOST110-2320-B-006-035, and MOST109-2320-B-006-064 from the Ministry of Science and Technology of Taiwan.
Author information
Authors and Affiliations
Contributions
JYL and CHC performed experiments and analyzed data. CLS designed some experiments and wrote the manuscript. YJY designed some experiments and analyzed some data. YQS performed some fluorescence experiments. CHC and PWG supervised JYL’s works, and PWG supervised YJY. CHC and PWG designed experiments and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, JY., Yu, YJ., Su, CL. et al. Modulation of methamphetamine memory reconsolidation by neural projection from basolateral amygdala to nucleus accumbens. Neuropsychopharmacol. 48, 478–488 (2023). https://doi.org/10.1038/s41386-022-01417-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-022-01417-y
This article is cited by
-
Dorsal raphe to basolateral amygdala corticotropin-releasing factor circuit regulates cocaine-memory reconsolidation
Neuropsychopharmacology (2024)