Abstract
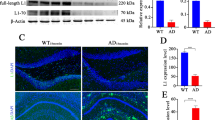
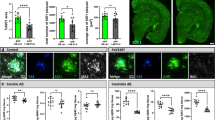
The approval of anti-amyloid β (Aβ) monoclonal antibodies (lecanemab) for the treatment of patients with early preclinical stage of Alzheimer’s disease (AD) by the Food and Drug Administration, suggests the reliability and importance of brain Aβ clearance for AD therapy. Microglia are the main phagocytes that clear Aβ in the brain, but the underlying regulatory mechanism is unclear. Here, we investigate the critical role of cathepsin B (CatB) in modulating microglial Aβ clearance from mouse brain. Wild-type or CatB−/− mice were injected with Aβ into the hippocampus from 1 to 3 weeks. Mice were evaluated for cognitive change, Aβ metabolism, neuroinflammation. Microglia and neuron cultures were prepared to verify the in vivo results. The statistical analyses were performed by student’s t test, one-way ANOVA with a post hoc Tukey’s test using the GraphPad Prism software package. CatB deficiency significantly reduces Aβ clearance efficiency and aggravates mouse cognitive decline. Exogenous Aβ markedly increases CatB expression in activated microglia. Transcriptome analysis and in vitro cell culture experiments demonstrate that CatB is associated with gene clusters involved in migration, phagocytosis, and inflammation. In addition, transcriptome analysis and immunoblotting suggest that CatB modulates microglial Aβ clearance via PI3K-AKT activation. Our study unveils a previously unknown role of CatB in promoting microglial functionality during Aβ clearance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data supporting the conclusions of this article are included within the article and the additional files.
References
Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–62.
van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388:9–21.
Ni J, Wu Z, Peterts C, Yamamoto K, Qing H, Nakanishi H. The critical role of proteolytic relay through cathepsins B and E in the phenotypic change of microglia/macrophage. J Neurosci. 2015;35:12488–501.
Butler CA, Popescu AS, Kitchener EJA, Allendorf DH, Puigdellivol M, Brown GC. Microglial phagocytosis of neurons in neurodegeneration, and its regulation. J Neurochem. 2021;158:621–39.
Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell. 2017;169:1276–90.e17.
Deczkowska A, Keren-Shaul H, Weiner A, Colonna M, Schwartz M, Amit I. Disease-associated microglia: a universal immune sensor of neurodegeneration. Cell. 2018;173:1073–81.
Barrett AJ. Human cathepsin B1. purification and some properties of the enzyme. Biochem J. 1973;131:809–22.
Xie Z, Zhao M, Yan C, Kong W, Lan F, Narengaowa, et al. Cathepsin B in programmed cell death machinery: mechanisms of execution and regulatory pathways. Cell Death Dis. 2023;14:255.
Wu Z, Ni J, Liu Y, Teeling JL, Takayama F, Collcutt A, et al. Cathepsin B plays a critical role in inducing Alzheimer’s disease-like phenotypes following chronic systemic exposure to lipopolysaccharide from Porphyromonas gingivalis in mice. Brain Behav Immun. 2017;65:350–61.
Sun B, Zhou Y, Halabisky B, Lo I, Cho SH, Mueller-Steiner S, et al. Cystatin C-cathepsin B axis regulates amyloid beta levels and associated neuronal deficits in an animal model of Alzheimer’s disease. Neuron. 2008;60:247–57.
Embury CM, Dyavarshetty B, Lu Y, Wiederin JL, Ciborowski P, Gendelman HE, et al. Cathepsin B improves ss-amyloidosis and learning and memory in models of Alzheimer’s disease. J Neuroimmune Pharmacol. 2017;12:340–52.
Liu Y, Wu Z, Zhang X, Ni J, Yu W, Zhou Y, et al. Leptomeningeal cells transduce peripheral macrophages inflammatory signal to microglia in reponse to Porphyromonas gingivalis LPS. Mediators Inflamm. 2013;2013:407562.
Ni J, Wu Z, Meng J, Saito T, Saido TC, Qing H, et al. An impaired intrinsic microglial clock system induces neuroinflammatory alterations in the early stage of amyloid precursor protein knock-in mouse brain. J Neuroinflammation. 2019;16:173.
Czirr E, Castello NA, Mosher KI, Castellano JM, Hinkson IV, Lucin KM, et al. Microglial complement receptor 3 regulates brain Aβ levels through secreted proteolytic activity. J Exp Med. 2017;214:1081–92.
Xie Z, Meng J, Wu Z, Nakanishi H, Hayashi Y, Kong W, et al. The dual nature of microglia in Alzheimer’s disease: a microglia-neuron crosstalk perspective. Neuroscientist. 2022;29:616–38.
Stoka V, Turk V, Turk B. Lysosomal cathepsins and their regulation in aging and neurodegeneration. Ageing Res Rev. 2016;32:22–37.
Ni J, Wu Z, Stoka V, Meng J, Hayashi Y, Peters C, et al. Increased expression and altered subcellular distribution of cathepsin B in microglia induce cognitive impairment through oxidative stress and inflammatory response in mice. Aging Cell. 2019;18:e12856.
Saito T, Matsuba Y, Mihira N, Takano J, Nilsson P, Itohara S, et al. Single App knock-in mouse models of Alzheimer’s disease. Nat Neurosci. 2014;17:661–3.
Perego C, Fumagalli S, De Simoni MG. Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. J Neuroinflammation. 2011;8:174.
Uxa S, Castillo-Binder P, Kohler R, Stangner K, Muller GA, Engeland K. Ki-67 gene expression. Cell Death Differ. 2021;28:3357–70.
Chu E, Mychasiuk R, Hibbs ML, Semple BD. Dysregulated phosphoinositide 3-kinase signaling in microglia: shaping chronic neuroinflammation. J Neuroinflammation. 2021;18:276.
Zhao Y, Wu X, Li X, Jiang L-L, Gui X, Liu Y, et al. TREM2 is a receptor for β-amyloid that mediates microglial function. Neuron. 2018;97:1023–31.
Matosin N, Fernandez-Enright F, Lum JS, Engel M, Andrews JL, Gassen NC, et al. Molecular evidence of synaptic pathology in the CA1 region in schizophrenia. NPJ Schizophr. 2016;2:16022.
Hook VY, Kindy M, Hook G. Inhibitors of cathepsin B improve memory and reduce beta-amyloid in transgenic Alzheimer disease mice expressing the wild-type, but not the Swedish mutant, beta-secretase site of the amyloid precursor protein. J Biol Chem. 2008;283:7745–53.
Kindy MS, Yu J, Zhu H, El-Amouri SS, Hook V, Hook GR. Deletion of the cathepsin B gene improves memory deficits in a transgenic ALZHeimer’s disease mouse model expressing AbetaPP containing the wild-type beta-secretase site sequence. J Alzheimers Dis. 2012;29:827–40.
Hook VY, Kindy M, Reinheckel T, Peters C, Hook G. Genetic cathepsin B deficiency reduces beta-amyloid in transgenic mice expressing human wild-type amyloid precursor protein. Biochem Biophys Res Commun. 2009;386:284–8.
Moon HY, Becke A, Berron D, Becker B, Sah N, Benoni G, et al. Running-induced systemic cathepsin B secretion is associated with memory function. Cell Metab. 2016;24:332–40.
Monobe M, Katayanagi Y, Maeda-Yamamoto M, Hiramoto S. Enhancement of the immunostimulatory activity of 1,25-dihydroxyvitamin D3-differentiated HL60 cells with an arabinoxylan from wheat bran. Food Sci Technol Res. 2012;18:481–84.
Njie EG, Boelen E, Stassen FR, Steinbusch HW, Borchelt DR, Streit WJ. Ex vivo cultures of microglia from young and aged rodent brain reveal age-related changes in microglial function. Neurobiol Aging. 2012;33:195.e1–12.
Floden AM, Combs CK. Microglia demonstrate age-dependent interaction with amyloid-beta fibrils. J Alzheimers Dis. 2011;25:279–93.
Daria A, Colombo A, Llovera G, Hampel H, Willem M, Liesz A, et al. Young microglia restore amyloid plaque clearance of aged microglia. EMBO J. 2017;36:583–603.
Tarassishin L, Suh HS, Lee SC. Interferon regulatory factor 3 plays an anti-inflammatory role in microglia by activating the PI3K/Akt pathway. J Neuroinflammation. 2011;8:187.
Xu ZZ, Xiu P, Lv JW, Wang FH, Dong XF, Liu F, et al. Integrin alphavbeta3 is required for cathepsin B-induced hepatocellular carcinoma progression. Mol Med Rep. 2015;11:3499–504.
Jiang M, Meng J, Zeng F, Qing H, Hook G, Hook V, et al. Cathepsin B inhibition blocks neurite outgrowth in cultured neurons by regulating lysosomal trafficking and remodeling. J Neurochem. 2020;155:300–12.
Elmore MRP, Hohsfield LA, Kramár EA, Soreq L, Lee RJ, Pham ST, et al. Replacement of microglia in the aged brain reverses cognitive, synaptic, and neuronal deficits in mice. Aging Cell. 2018;17:e12832.
Meng J, Liu Y, Xie Z, Qing H, Lei P, Ni J. Nucleus distribution of cathepsin B in senescent microglia promotes brain aging through degradation of sirtuins. Neurobiol Aging. 2020;96:255–66.
Ni J, Lan F, Xu Y, Nakanishi H, Li X. Extralysosomal cathepsin B in central nervous system: mechanisms and therapeutic implications. Brain Pathol. 2022;32:e13071.
Babcock AA, Ilkjaer L, Clausen BH, Villadsen B, Dissing-Olesen L, Bendixen AT, et al. Cytokine-producing microglia have an altered beta-amyloid load in aged APP/PS1 Tg mice. Brain Behav Immun. 2015;48:86–101.
Rivera-Escalera F, Pinney JJ, Owlett L, Ahmed H, Thakar J, Olschowka JA, et al. IL-1beta-driven amyloid plaque clearance is associated with an expansion of transcriptionally reprogrammed microglia. J Neuroinflammation. 2019;16:261.
Acknowledgements
We thank the Biological and Medical Engineering Core Facilities of Beijing Institute of Technology for supporting experimental equipment.
Funding
This work was supported by funding from National Natural Science Foundation of China (32070954, 82401130, 82101394, 82001167), Guangdong Provincial Department of Education General University Innovation Team Project (2024KCXTD016), Beijing Natural Science Foundation (7212066) and China Postdoctoral Science Foundation (2023MD734244).
Author information
Authors and Affiliations
Contributions
JN designed the research and wrote the manuscript; MJ performed most of the experiments and data analysis and wrote the manuscript. DZ, YZ, WK, and ZX performed part of the animal experiments. YX and YL conducted cell culture. SZ, XK, SZ, and RM analyzed data. YP, ZW, HN, JZ, HL, and ZQ provided the reagents. LL and HQ provided helpful suggestions to the manuscript. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The author declares no competing interests.
Ethics approval
All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee of the Beijing Institute of Technology (BIT-EC-SCXK2018-0003-M-2021028) in conformity with the international guidelines on the ethics of animal experimentation.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, M., Zhao, D., Zhou, Y. et al. Cathepsin B modulates microglial migration and phagocytosis of amyloid β in Alzheimer’s disease through PI3K-Akt signaling. Neuropsychopharmacol. 50, 640–650 (2025). https://doi.org/10.1038/s41386-024-01994-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-024-01994-0
This article is cited by
-
Differential downstream signaling in microglia lacking Alzheimer’s-related TREM2 or its adaptor TYROBP/DAP12
Molecular Neurodegeneration Advances (2026)
-
Microglial phagocytosis in Alzheimer disease
Nature Reviews Neurology (2026)