Abstract
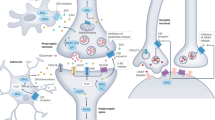
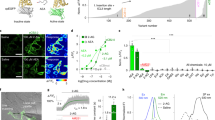
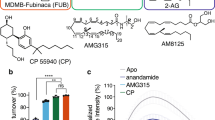
Up to 45% of patients surviving from transient global cerebral ischemia (GCI) after cardiac arrest develop post-global cerebral ischemia depression (PGCID), but how to treat PGCID is clinically unknown. Here we find that cannabinoid type-1 receptor (CB1R) antagonists, CB1R knockout and endocannabinoid (eCB) synthesis inhibition block acute stress-induced PGCID. Application of acute stress to GCI mice increases CB1R activity from ventromedial prefrontal cortical (vmPFC) terminals synapsing with the basolateral amygdala (BLA) neurons, indicating the involvement of increased vmPFC-BLA synaptic eCB signaling in PGCID induction. This idea is supported by findings that optogenetic activation of CB1Rs in vmPFC-BLA projections mimics stress effects to induce PGCID, which is blocked by knock-down of eCB biosynthesis enzyme genes in vmPFC-BLA synapses. Interestingly, GCI mice show decreased mRNA expression of eCB degradation enzymes in vmPFCs without significant changes on mRNA expression of eCB biosynthesis and degradation enzymes in BLA cells. Thus, over-expression of eCB degradation enzymes in vmPFC cells innervating BLA neurons or activation of vmPFC-BLA projections blocks stress effects to induce PGCID. Our findings suggest that decreased eCB degradation and subsequent stress-increased eCB signaling in vmPFC-BLA circuits participate in the mechanism of PGCID, which can be treated clinically by eCB signaling interference systemically or in vmPFC-BLA circuits.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Zheng ZJ, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104:2158–63.
Bigham BL, Koprowicz K, Rea T, Dorian P, Aufderheide TP, Davis DP, et al. Cardiac arrest survival did not increase in the Resuscitation Outcomes Consortium after implementation of the 2005 AHA CPR and ECC guidelines. Resuscitation. 2011;82:979–83.
Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300:1423–31.
Segal N, di Pompéo C, Escutnaire J, Wiel E, Dumont C, Castra L, et al. Evolution of survival in cardiac arrest with age in elderly patients: is resuscitation a dead end? J Emerg Med. 2018;54:295–301.
Roine RO, Kajaste S, Kaste M. Neuropsychological sequelae of cardiac arrest. JAMA. 1993;269:237–42.
Wilder Schaaf KP, Artman LK, Peberdy MA, Walker WC, Ornato JP, Gossip MR, et al. Anxiety, depression, and PTSD following cardiac arrest: a systematic review of the literature. Resuscitation. 2013;84:873–7.
Metzger K, Gamp M, Tondorf T, Hochstrasser S, Becker C, Luescher T, et al. Depression and anxiety in relatives of out-of-hospital cardiac arrest patients: results of a prospective observational study. J Crit Care. 2019;51:57–63.
Yaow CYL, Teoh SE, Lim WS, Wang R, Han MX, Pek PP, et al. Prevalence of anxiety, depression, and post-traumatic stress disorder after cardiac arrest: A systematic review and meta-analysis. Resuscitation. 2022;170:82–91.
Raic M. Depression and heart diseases: leading health problems. Psychiatr Danub. 2017;29:770–7.
de la Tremblaye PB, Linares NN, Schock S, Plamondon H. Activation of CRHR1 receptors regulates social and depressive-like behaviors and expression of BDNF and TrkB in mesocorticolimbic regions following global cerebral ischemia. Exp Neurol. 2016;284:84–97.
Yan B, Wang DY, Xing DM, Ding Y, Wang RF, Lei F, Du LJ. The antidepressant effect of ethanol extract of radix puerariae in mice exposed to cerebral ischemia reperfusion. Pharmacol Biochem Behav. 2004;78:319–25.
Yan B, He J, Xu H, Zhang Y, Bi X, Thakur S, et al. Quetiapine attenuates the depressive and anxiolytic-like behavioural changes induced by global cerebral ischemia in mice. Behav Brain Res. 2007;182:36–41.
Camargos QM, Silva BC, Silva DG, Toscano E, Oliveira B, Bellozi P, et al. Minocycline treatment prevents depression and anxiety-like behaviors and promotes neuroprotection after experimental ischemic stroke. Brain Res Bull. 2020;155:1–10.
Wang Y, Gu N, Duan T, Kesner P, Blaskovits F, Liu J, et al. Monoacylglycerol lipase inhibitors produce pro- or antidepressant responses via hippocampal CA1 GABAergic synapses. Mol Psychiatry. 2017;22:215–26.
Hill MN, Gorzalka BB. Pharmacological enhancement of cannabinoid CB1 receptor activity elicits an antidepressant-like response in the rat forced swim test. Eur Neuropsychopharmacol. 2005;15:593–9.
Bambico FR, Katz N, Debonnel G, Gobbi G. Cannabinoids elicit antidepressant-like behavior and activate serotonergic neurons through the medial prefrontal cortex. J Neurosci. 2007;27:11700–11.
Jiang W, Zhang Y, Xiao L, Van Cleemput J, Ji SP, Bai G, Zhang X. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J Clin Investig. 2005;115:3104–16.
Zhong P, Wang W, Pan B, Liu X, Zhang Z, Long JZ, et al. Monoacylglycerol lipase inhibition blocks chronic stress-induced depressive-like behaviors via activation of mTOR signaling. Neuropsychopharmacology. 2014;39:1763–76.
Alteba S, Mizrachi Zer-Aviv T, Tenenhaus A, Ben David G, Adelman J, Hillard CJ, et al. Antidepressant-like effects of URB597 and JZL184 in male and female rats exposed to early life stress. Eur Neuropsychopharmacol. 2020;39:70–86.
Han J, Kesner P, Metna-Laurent M, Duan T, Xu L, Georges F, et al. Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell. 2012;148:1039–50.
Blankman JL, Cravatt BF. Chemical probes of endocannabinoid metabolism. Pharmacol Rev. 2013;65:849–71.
Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76.
Katona I, Sperlágh B, Sík A, Käfalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–58.
Melis M, Pistis M, Perra S, Muntoni AL, Pillolla G, Gessa GL. Endocannabinoids mediate presynaptic inhibition of glutamatergic transmission in rat ventral tegmental area dopamine neurons through activation of CB1 receptors. J Neurosci. 2004;24:53–62.
Mecca CM, Chao D, Yu G, Feng Y, Segel I, Zhang Z, et al. Dynamic change of endocannabinoid signaling in the medial prefrontal cortex controls the development of depression after neuropathic pain. J Neurosci. 2021;41:7492–508.
Jenniches I, Ternes S, Albayram O, Otte DM, Bach K, Bindila L, et al. Anxiety, Stress, and Fear Response in Mice With Reduced Endocannabinoid Levels. Biol Psychiatry. 2016;79:858–68.
Shen CJ, Zheng D, Li KX, Yang JM, Pan HQ, Yu XD, et al. Cannabinoid CB(1) receptors in the amygdalar cholecystokinin glutamatergic afferents to nucleus accumbens modulate depressive-like behavior. Nat Med. 2019;25:337–49.
Steiner MA, Marsicano G, Wotjak CT, Lutz B. Conditional cannabinoid receptor type 1 mutants reveal neuron subpopulation-specific effects on behavioral and neuroendocrine stress responses. Psychoneuroendocrinology. 2008;33:1165–70.
Zhong H, Tong L, Gu N, Gao F, Lu Y, Xie RG, et al. Endocannabinoid signaling in hypothalamic circuits regulates arousal from general anesthesia in mice. J Clin Invest. 2017;127:2295–309.
Onken M, Berger S, Kristian T. Simple model of forebrain ischemia in mouse. J Neurosci Methods. 2012;204:254–61.
Charney, DS, Nestler EJ. Neurobiology of mental illness. New York: Oxford University Press; 2005.
Hare BD, Shinohara R, Liu RJ, Pothula S, DiLeone RJ, Duman RS. Optogenetic stimulation of medial prefrontal cortex Drd1 neurons produces rapid and long-lasting antidepressant effects. Nat Commun. 2019;10:223.
Dong A, He K, Dudok B, Farrell JS, Guan W, Liput DJ, et al. A fluorescent sensor for spatiotemporally resolved imaging of endocannabinoid dynamics in vivo. Nat Biotechnol. 2022;40:787–98.
Shang H, Li P, Lin X, Cai Q, Li Z, Deng L, et al. Neuronal and astrocytic CB1R signaling differentially modulates goal-directed behavior and working memory by distinct temporal mechanisms. Neuropsychopharmacology. 2023;48:1520–31.
Ran FA, Cong L, Yan WX, Scott DA, Gootenberg JS, Kriz AJ, et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature. 2015;520:186–91.
Xie H, Tang L, He X, Liu X, Zhou C, Liu J, et al. SaCas9 requires 5’-NNGRRT-3’ PAM for sufficient cleavage and possesses higher cleavage activity than SpCas9 or FnCpf1 in human cells. Biotechnol J. 2018;13:1800080.
Sun H, Fu S, Cui S, Yin X, Sun X, Qi X, et al. Development of a CRISPR-SaCas9 system for projection- and function-specific gene editing in the rat brain. Sci Adv. 2020;6:eaay6687.
Gunduz-Cinar O, Hill MN, McEwen BS, Holmes A. Amygdala FAAH and anandamide: mediating protection and recovery from stress. Trends Pharmacol Sci. 2013;34:637–44.
Zou S, Kumar U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int J Mol Sci. 2018;19:833.
Hill MN, Miller GE, Ho WS, Gorzalka BB, Hillard CJ. Serum endocannabinoid content is altered in females with depressive disorders: a preliminary report. Pharmacopsychiatry. 2008;41:48–53.
Haring M, Guggenhuber S, Lutz B. Neuronal populations mediating the effects of endocannabinoids on stress and emotionality. Neuroscience. 2012;204:145–58.
Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–87.
Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, et al. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb Cortex. 2014;24:2981–90.
Kral TRA, Schuyler BS, Mumford JA, Rosenkranz MA, Lutz A, Davidson RJ. Impact of short- and long-term mindfulness meditation training on amygdala reactivity to emotional stimuli. Neuroimage. 2018;181:301–13.
Little JP, Carter AG. Synaptic mechanisms underlying strong reciprocal connectivity between the medial prefrontal cortex and basolateral amygdala. J Neurosci. 2013;33:15333–42.
McGarry LM, Carter AG. Prefrontal Cortex Drives Distinct Projection Neurons in the Basolateral Amygdala. Cell Rep. 2017;21:1426–33.
Arruda-Carvalho M, Clem RL. Pathway-selective adjustment of prefrontal-amygdala transmission during fear encoding. J Neurosci. 2014;34:15601–9.
Bloodgood DW, Sugam JA, Holmes A, Kash TL. Fear extinction requires infralimbic cortex projections to the basolateral amygdala. Transl Psychiatry. 2018;8:60.
McGinnis MM, Parrish BC, Chappell AM, Alexander NJ, McCool BA. Chronic Ethanol Differentially Modulates Glutamate Release from Dorsal and Ventral Prefrontal Cortical Inputs onto Rat Basolateral Amygdala Principal Neurons. eNeuro. 2020;7:ENEURO.0132-19.2019.
Funding
The Major program of Technological innovation 2030 “Brain science and brain-inspired research” (2021ZD0203002); Key Project from the National Natural Science Foundation of China (81830035); The Major program of National Natural Science Foundation of China (82090033); The Major Basic research program of Shandong Province Natural Science Foundation (ZR2019ZD35); National Natural Science Foundation of China (32371187,32000788); Natural Science Foundation of Shandong Province (ZR2024MH063).exte.
Author information
Authors and Affiliations
Contributions
XX, ZT, YM, FG, HX, HS, SZ, BX, YQ and NZ performed the research; XZ and XX analyzed data; XZ conceived the project and wrote the manuscript with the help of XX, JC, YL, and JH.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tu, Z., Ma, Y., Shang, H. et al. Endocannabinoid interference blocks post-global cerebral ischemia depression through prefrontal cortico-amygdala projections. Neuropsychopharmacol. 50, 1063–1074 (2025). https://doi.org/10.1038/s41386-024-02029-4
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-024-02029-4