Abstract
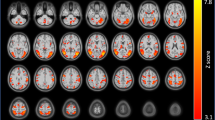
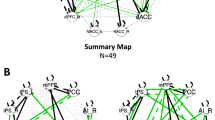
Individual differences in brain intrinsic functional connectivity (FC) and reactivity to nicotine cues are linked to variability in clinical outcomes in nicotine dependence. However, the relative contributions and potential interdependencies of these brain imaging-derived phenotypes in the context of craving and nicotine dependence are unclear. Moreover, it is unknown whether these relationships differ in individuals who smoke versus vape nicotine. To investigate these questions, eighty-six individuals who use nicotine daily (n = 67 smoking, n = 19 vaping) completed either a smoking or vaping cue-reactivity task and a resting-state scan during functional magnetic resonance imaging (fMRI). Validating the efficacy of the smoking and vaping tasks, both cohorts displayed robust reactivity to nicotine versus neutral cues in the default mode network (DMN) and the anterior insula (AI), a primary node of the salience network (SN), which did not habituate over time. In the smoking and vaping groups, lower prefrontal reactivity to nicotine versus neutral cues and greater resting-state FC between nodes of the SN and DMN were associated with higher cue-induced craving. Moreover, we found that the former partially mediated the latter, suggesting a mechanism in which high resting SN-DMN connectivity increases craving susceptibility partly via a constraining effect on regulatory prefrontal reactivity to cues. These relationships were not impacted by group, suggesting that links between brain function and craving are similar regardless of smoking or vaping nicotine.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets analyzed during the current study are available upon reasonable request.
References
Betts JM, Dowd AN, Forney M, Hetelekides E, Tiffany ST. A meta-analysis of cue reactivity in tobacco cigarette smokers. Nicotine Tob Res. 2021;23:249–58.
Ekhtiari H, Zare-Bidoky M, Sangchooli A, Janes AC, Kaufman MJ, Oliver JA, et al. A methodological checklist for fMRI drug cue reactivity studies: development and expert consensus. Nat Protoc. 2022;17:567–95.
Allenby C, Falcone M, Wileyto EP, Cao W, Bernardo L, Ashare RL, et al. Neural cue reactivity during acute abstinence predicts short-term smoking relapse. Addict Biol. 2020;25:e12733.
Janes A, Gilman J, Radoman M, Pachas G, Fava M, Evins A. Revisiting the role of the insula and smoking cue-reactivity in relapse: a replication and extension of neuroimaging findings. Drug Alcohol Depend. 2017;179:8–12.
Janes AC, Krantz NL, Nickerson LD, Frederick BB, Lukas SE. Craving and cue reactivity in nicotine-dependent tobacco smokers is associated with different insula networks. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:76–83.
Janes AC, Pizzagalli DA, Richardt S, Frederick BD, Chuzi S, Pachas G, et al. Brain Reactivity to Smoking Cues Prior to Smoking Cessation Predicts Ability to Maintain Tobacco Abstinence. Biol Psychiatry. 2010;67:722–29.
National Center for Health Statistics. National Health Interview Survey. 2024. https://www.cdc.gov/nchs/products/databriefs/db524.htm#:~:text=The%20percentage%20of%20adults%20age,Figure%201%2C%20Table%201.
Foxon F, Selya A, Gitchell J, Shiffman S. Increased e-cigarette use prevalence is associated with decreased smoking prevalence among US adults. Harm Reduct J. 2024;21:136.
Vafaie N, Kober H. Association of drug cues and craving with drug use and relapse: a systematic review and meta-analysis. JAMA psychiatry. 2022;79:641–50.
Conklin CA, McClernon FJ, Vella EJ, Joyce CJ, Salkeld RP, Parzynski CS, et al. Combined smoking cues enhance reactivity and predict immediate subsequent smoking. Nicotine Tob Res. 2019;21:241–48.
McClernon FJ, Hiott FB, Liu J, Salley AN, Behm FM, Rose JE. IMAGING STUDY: Selectively reduced responses to smoking cues in amygdala following extinction‐based smoking cessation: results of a preliminary functional magnetic resonance imaging study. Addiction Biol. 2007;12:503–12.
Lin X, Deng J, Shi L, Wang Q, Li P, Li H, et al. Neural substrates of smoking and reward cue reactivity in smokers: a meta-analysis of fMRI studies. Transl psychiatry. 2020;10:97.
Wang KS, Kaiser RH, Peechatka AL, Frederick BB, Janes AC. Temporal dynamics of large-scale networks predict neural cue reactivity and cue-induced craving. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:1011–18.
Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38.
Menon V. 20 years of the default mode network: A review and synthesis. Neuron. 2023;111:2469–87.
Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–67.
Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56.
Gu X, Lohrenz T, Salas R, Baldwin PR, Soltani A, Kirk U, et al. Belief about nicotine modulates subjective craving and insula activity in deprived smokers. Front psychiatry. 2016;7:126.
Regner MF, Tregellas J, Kluger B, Wylie K, Gowin JL, Tanabe J. The insula in nicotine use disorder: functional neuroimaging and implications for neuromodulation. Neurosci Biobehav Rev. 2019;103:414–24.
Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–34.
Joutsa J, Moussawi K, Siddiqi SH, Abdolahi A, Drew W, Cohen AL, et al. Brain lesions disrupting addiction map to a common human brain circuit. Nat Med. 2022;28:1249–55.
Janes AC, Farmer S, Peechatka AL, Frederick BDB, Lukas SE. Insula–Dorsal Anterior Cingulate Cortex Coupling is Associated with Enhanced Brain Reactivity to Smoking Cues. Neuropsychopharmacology. 2015;40:1561–68.
Murray L, Frederick BB, Janes AC. Data-driven connectivity profiles relate to smoking cessation outcomes. Neuropsychopharmacology. 2024;49:1007–13.
Sutherland MT, Carroll AJ, Salmeron BJ, Ross TJ, Hong LE, Stein EA. Down-regulation of amygdala and insula functional circuits by varenicline and nicotine in abstinent cigarette smokers. Biol Psychiatry. 2013;74:538–46.
Ekhtiari H, Kuplicki R, Aupperle RL, Paulus MP. It is never as good the second time around: Brain areas involved in salience processing habituate during repeated drug cue exposure in treatment engaged abstinent methamphetamine and opioid users. NeuroImage. 2021;238:118180.
Franklin T, Wang Z, Suh JJ, Hazan R, Cruz J, Li Y, et al. Effects of Varenicline on Smoking Cue–Triggered Neural and Craving Responses. Arch Gen Psychiatry. 2011;68:516–26.
Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Br J Addiction. 1991;86:1467–76.
Korponay C, Janes AC, Frederick BB. Brain-wide functional connectivity artifactually inflates throughout functional magnetic resonance imaging scans. Nat Hum Behav. 2024;8:1568–80.
Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci. 2009;106:13040–45.
Faillenot I, Heckemann RA, Frot M, Hammers A. Macroanatomy and 3D probabilistic atlas of the human insula. Neuroimage. 2017;150:88–98.
Hayes AF. PROCESS: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling. KS; University of Kansas: 2012.
Janes AC, Betts J, Jensen JE, Lukas SE. Dorsal anterior cingulate glutamate is associated with engagement of the default mode network during exposure to smoking cues. Drug Alcohol Depend. 2016;167:75–81.
Janes AC, Gilman JM, Radoman M, Pachas G, Fava M, Evins AE. Revisiting the role of the insula and smoking cue-reactivity in relapse: A replication and extension of neuroimaging findings. Drug Alcohol Depend. 2017;179:8–12.
Koban L, Wager TD, Kober H. A neuromarker for drug and food craving distinguishes drug users from non-users. Nat Neurosci. 2023;26:316–25.
Beaglehole R, Bates C, Youdan B, Bonita R. Nicotine without smoke: fighting the tobacco epidemic with harm reduction. Lancet. 2019;394:718–20.
Lerman C, Gu H, Loughead J, Ruparel K, Yang Y, Stein EA. Large-scale brain network coupling predicts acute nicotine abstinence effects on craving and cognitive function. JAMA psychiatry. 2014;71:523–30.
Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, et al. Prefrontal–striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci. 2010;107:14811–16.
Cole MW, Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions. Neuroimage. 2007;37:343–60.
Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–69.
Maiti R, Mishra BR, Hota D. Effect of high-frequency transcranial magnetic stimulation on craving in substance use disorder: a meta-analysis. J Neuropsychiatry Clin Neurosci. 2017;29:160–71.
Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci. 2008;105:12569–74.
Funding
This research was supported by the National Institutes on Drug Abuse R01DA039135 and the Intramural Research Program of the NIH, NIDA.
Author information
Authors and Affiliations
Contributions
LM, BBF, SEL, CK, and ACJ contributed to the design and implementation of the research, to the analysis of the results, and to the writing of the manuscript. MKS contributed to the analysis of the results and to the writing of the manuscript
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Murray, L., Scavnicky, M.K., Korponay, C. et al. Brain reactivity to nicotine cues mediates the link between resting-state connectivity and cue-induced craving in individuals who smoke or vape nicotine. Neuropsychopharmacol. 50, 983–990 (2025). https://doi.org/10.1038/s41386-025-02083-6
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-025-02083-6
This article is cited by
-
From rest to focus: pharmacological modulation of the relationship between resting state dorsal attention network dynamics and task-based brain activation
Neuropsychopharmacology (2026)
-
Cue labeling reduces cigarette craving and associated neural activity
Neuropsychopharmacology (2025)