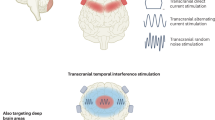
Abstract
Non-invasive Brain Stimulation (NIBS) technologies, including transcranial electrical (tES) and magnetic (TMS) stimulation, have emerged as promising interventions for various psychiatric disorders. FDA-approved TMS protocols in depression, OCD and nicotine use disorder provide a meaningful improvement. Treatment efficacy however remains inconsistent across individuals, and one relevant reason is intervention effect variability based on individual factors. There is a growing effort to develop individualized interventions, reinforced recently by FDA approval of a new TMS protocol that includes individualized fMRI-based targeting along with other modifications with higher reported effect size than previous “one size fits all” protocols. This paper discusses the dimensions for individualizing tES/TMS protocols to enhance therapeutic efficacy. We propose a multifaceted approach to personalizing NIBS, considering four levels: (1) context, (2) target, (3) dose, and (4) timing. By addressing inter- and intra-individual variability, we highlight a path toward precision medicine using individualized Brain Stimulation to treat psychiatric diseases. Despite challenges and limitations, this approach encourages broader and more systematic adoption of personalized Brain Stimulation techniques to improve clinical outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Change history
19 May 2025
The original online version of this article was revised: The copyright holder for this article was incorrectly given as ‘This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply 2025’ but should have been ‘The Author(s), under exclusive licence to American College of Neuropsychopharmacology 2025’.
18 June 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41386-025-02134-y
References
Cohen SL, Bikson M, Badran BW, George MS. A visual and narrative timeline of US FDA milestones for Transcranial Magnetic Stimulation (TMS) devices. Brain Stimul. 2022;15:73–5.
Cocchi L, Zalesky A. Personalized transcranial magnetic stimulation in psychiatry. Biol Psychiatry. 2018;3:731–41.
Cole EJ, Phillips AL, Bentzley BS, Stimpson KH, Nejad R, Barmak F, et al. Stanford neuromodulation therapy (SNT): a double-blind randomized controlled trial. Am J Psychiatry. 2022;179:132–41.
Steele VR, Maxwell AM. Treating cocaine and opioid use disorder with transcranial magnetic stimulation: a path forward. Pharmacol Biochem Behav. 2021;209:173240.
Ekhtiari H, Tavakoli H, Addolorato G, Baeken C, Bonci A, Campanella S, et al. Transcranial electrical and magnetic stimulation (tES and TMS) for addiction medicine: a consensus paper on the present state of the science and the road ahead. Neurosci Biobehav Rev. 2019;104:118–40.
Maier M Personalized medicine—a tradition in general practice! : Taylor & Francis; 2019. p. 63-4.
Lulic T, El-Sayes J, Fassett HJ, Nelson AJ. Physical activity levels determine exercise-induced changes in brain excitability. PloS one. 2017;12:e0173672.
Cabrera L, Achtyes E, Bluhm R, McCright A. Views about neuromodulation interventions for depression by stakeholder group, treatment modality, and depression severity. Compr Psychiatry. 2023;122:152365.
Sullivan CR, Henry A, Lehman J, Caola L, Nahas Z, Widge AS, et al. Rewriting the script: the need for effective education to address racial disparities in transcranial magnetic stimulation uptake in BIPOC communities. Neuroethics. 2024;17:8.
Kricheldorff J, Göke K, Kiebs M, Kasten FH, Herrmann CS, Witt K, et al. Evidence of neuroplastic changes after transcranial magnetic, electric, and deep Brain Stimulation. Brain Sci. 2022;12:929.
Spagnolo PA, Montemitro C, Pettorruso M, Martinotti G, Di Giannantonio M. Better together? Coupling pharmacotherapies and cognitive interventions with non-invasive Brain Stimulation for the treatment of addictive disorders. Front Neurosci. 2020;13:1385.
Sathappan AV, Luber BM, Lisanby SH. The dynamic duo: combining noninvasive Brain Stimulation with cognitive interventions. Prog Neuro-Psychopharmacol Biol Psychiatry. 2019;89:347–60.
Xu X, Ding X, Chen L, Chen T, Su H, Li X, et al. The transcranial direct current stimulation over prefrontal cortex combined with the cognitive training reduced the cue-induced craving in female individuals with methamphetamine use disorder: a randomized controlled trial. J Psychiatr Res. 2021;134:102–10.
Hauer L, Scarano GI, Brigo F, Golaszewski S, Lochner P, Trinka E, et al. Effects of repetitive transcranial magnetic stimulation on nicotine consumption and craving: a systematic review. Psychiatry Res. 2019;281:112562.
Tofighi B, Chemi C, Ruiz-Valcarcel J, Hein P, Hu L. Smartphone apps targeting alcohol and illicit substance use: systematic search in in commercial app stores and critical content analysis. JMIR Mhealth Uhealth. 2019;7:e11831.
Cassani R, Novak GS, Falk TH, Oliveira AA. Virtual reality and non-invasive Brain Stimulation for rehabilitation applications: a systematic review. J Neuroeng Rehabil. 2020;17:1–16.
Kumpf U, Palm U, Eder J, Ezim H, Stadler M, Burkhardt G, et al. TDCS at home for depressive disorders: an updated systematic review and lessons learned from a prematurely terminated randomized controlled pilot study. Eur Arch Psychiatry Clin Neurosci. 2023;273:1403–20.
Sack AT, Paneva J, Küthe T, Dijkstra E, Zwienenberg L, Arns M, et al. Target engagement and brain state dependence of transcranial magnetic stimulation: implications for clinical practice. Biol Psychiatry. 2024;95:536–44.
Bradley C, Nydam AS, Dux PE, Mattingley JB. State-dependent effects of neural stimulation on brain function and cognition. Nat Rev Neurosci. 2022;23:459–75.
Schutter DJ, Smits F, Klaus J. Mind matters: A narrative review on affective state-dependency in non-invasive Brain Stimulation. Int J Clin Health Psychol. 2023;23:100378.
Koban L, Wager TD, Kober H. A neuromarker for drug and food craving distinguishes drug users from non-users. Nat Neurosci. 2023;26:316–25.
Kearney-Ramos TE, Dowdle LT, Mithoefer OJ, Devries W, George MS, Hanlon CA. State-dependent effects of ventromedial prefrontal cortex continuous thetaburst stimulation on cocaine cue reactivity in chronic cocaine users. Front Psychiatry. 2019;10:317.
Steele VR. Transcranial magnetic stimulation as an interventional tool for addiction. Front Neurosci. 2020;14:592343.
Zangen A, Moshe H, Martinez D, Barnea‐Ygael N, Vapnik T, Bystritsky A, et al. Repetitive transcranial magnetic stimulation for smoking cessation: a pivotal multicenter double‐blind randomized controlled trial. World Psychiatry. 2021;20:397–404.
Isserles M, Rosenberg O, Dannon P, Levkovitz Y, Kotler M, Deutsch F, et al. Cognitive–emotional reactivation during deep transcranial magnetic stimulation over the prefrontal cortex of depressive patients affects antidepressant outcome. J Affect Disord. 2011;128:235–42.
Ten Oever S, De Graaf TA, Bonnemayer C, Ronner J, Sack AT, Riecke L. Stimulus presentation at specific neuronal oscillatory phases experimentally controlled with tACS: Implementation and applications. Front Cell Neurosci. 2016;10:240.
Isserles M, Shalev AY, Roth Y, Peri T, Kutz I, Zlotnick E, et al. Effectiveness of deep transcranial magnetic stimulation combined with a brief exposure procedure in post-traumatic stress disorder–a pilot study. Brain Stimul. 2013;6:377–83.
Carmi L, Alyagon U, Barnea-Ygael N, Zohar J, Dar R, Zangen A. Clinical and electrophysiological outcomes of deep TMS over the medial prefrontal and anterior cingulate cortices in OCD patients. Brain Stimul. 2018;11:158–65.
Carmi L, Tendler A, Bystritsky A, Hollander E, Blumberger DM, Daskalakis J, et al. Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: a prospective multicenter randomized double-blind placebo-controlled trial. Am J Psychiatry. 2019;176:931–8.
Tendler A, Sisko E, DeLuca M, Garrison S, Mages D, Mayfield H, et al. Deep TMS for OCD with the H7 coil: Case series in a naturalistic setting. Brain Stimul. 2018;11:e14–e5.
Tendler A, Sisko E, Barnea-Ygael N, Zangen A, Storch EA. A method to provoke obsessive compulsive symptoms for basic research and clinical interventions. Front Psychiatry. 2019;10:814.
Hoogerwerf E, Greeven A, Goekoop R, Spinhoven P. Personalized exposure and experience sampling method feedback versus exposure as usual for obsessive–compulsive disorder: a study protocol for a randomized controlled trial. Trials. 2024;25:43.
Bikson M, Rahman A. Origins of specificity during tDCS: anatomical, activity-selective, and input-bias mechanisms. Front Hum Neurosci. 2013;7:688.
Nitsche MA, Liebetanz D, Antal A, Lang N, Tergau F, Paulus W. Modulation of cortical excitability by weak direct current stimulation–technical, safety and functional aspects. Suppl Clin Neurophysiol. 2003;56:255–76.
Ziemann U, Reis J, Schwenkreis P, Rosanova M, Strafella A, Badawy R, et al. TMS and drugs revisited 2014. Clin Neurophysiol. 2015;126:1847–68.
McDonnell MN, Orekhov Y, Ziemann U. Suppression of LTP-like plasticity in human motor cortex by the GABA B receptor agonist baclofen. Exp Brain Res. 2007;180:181–6.
Nitsche MA, Grundey J, Liebetanz D, Lang N, Tergau F, Paulus W. Catecholaminergic consolidation of motor cortical neuroplasticity in humans. Cereb Cortex. 2004;14:1240–5.
Martinotti G, Montemitro C, Pettorruso M, Viceconte D, Alessi M, Di Carlo F, et al. Augmenting pharmacotherapy with neuromodulation techniques for the treatment of bipolar disorder: a focus on the effects of mood stabilizers on cortical excitability. Expert Opin Pharmacother. 2019;20:1575–88.
Best SR, Pavel DG, Haustrup N. Combination therapy with transcranial magnetic stimulation and ketamine for treatment-resistant depression: a long-term retrospective review of clinical use. Heliyon. 2019;5:e02187.
Wrightson JG, Cole J, Sohn MN, McGirr A. The effects of D-cycloserine on corticospinal excitability after repeated spaced intermittent theta-burst transcranial magnetic stimulation: a randomized controlled trial in healthy individuals. Neuropsychopharmacology. 2023;48:1217–24.
Cole J, Sohn MN, Harris AD, Bray SL, Patten SB, McGirr A. Efficacy of adjunctive D-cycloserine to intermittent theta-burst stimulation for major depressive disorder: a randomized clinical trial. JAMA Psychiatry. 2022;79:1153–61.
Paulus W, Classen J, Cohen LG, Large CH, Di Lazzaro V, Nitsche M, et al. State of the art: pharmacologic effects on cortical excitability measures tested by transcranial magnetic stimulation. Brain Stimul. 2008;1:151–63.
Zhang M, Wang R, Luo X, Zhang S, Zhong X, Ning Y, et al. Repetitive transcranial magnetic stimulation target location methods for depression. Front Neurosci. 2021;15:695423.
Upton S, Brown AA, Ithman M, Newman-Norlund R, Sahlem G, Prisciandaro JJ, et al. Effects of hyperdirect pathway theta burst transcranial magnetic stimulation on inhibitory control, craving, and smoking in adults with nicotine dependence: A double-blind, randomized crossover trial. Biol Psychiatry 2023;8:1156–65.
Soleimani G, Kuplicki R, Lim K, Paulus M, Ekhtiari H, editors. Dual Neuromodulation Targets for Treatment of Substance Use Disorders: Unraveling the Interacting Role of DLPFC and Frontopolar Cortex During Drug Cue Reactivity. Neuropsychopharmacology; 2023: Springernature Campus, 4 Crinan St, London, N1 9xw, England.
Soleimani G, Conelea CA, Kuplicki R, Opitz A, Lim KO, Paulus MP, et al. Targeting VMPFC‐amygdala circuit with TMS in substance use disorder: a mechanistic framework. Addict Biol. 2025;30:e70011.
Ji GJ, Xie W, Yang T, Wu Q, Sui P, Bai T, et al. Pre‐supplementary motor network connectivity and clinical outcome of magnetic stimulation in obsessive–compulsive disorder. Hum Brain Mapp. 2021;42:3833–44.
Pedapati E, DiFrancesco M, Wu S, Giovanetti C, Nash T, Mantovani A, et al. Neural correlates associated with symptom provocation in pediatric obsessive compulsive disorder after a single session of sham-controlled repetitive transcranial magnetic stimulation. Psychiatry Res. 2015;233:466–73.
Szaflarski JP, Nenert R, Allendorfer JB, Martin AN, Amara AW, Griffis JC, et al. Intermittent theta burst stimulation (iTBS) for treatment of chronic post-stroke aphasia: results of a pilot randomized, double-blind, sham-controlled trial. Med Sci Monit. 2021;27:e931468–1.
Ren J, Ren W, Zhou Y, Dahmani L, Duan X, Fu X, et al. Personalized functional imaging-guided rTMS on the superior frontal gyrus for post-stroke aphasia: A randomized sham-controlled trial. Brain Stimul. 2023;16:1313–21.
Schönfeldt-Lecuona C, Grön G, Walter H, Büchler N, Wunderlich A, Spitzer M, et al. Stereotaxic rTMS for the treatment of auditory hallucinations in schizophrenia. Neuroreport. 2004;15:1669–73.
Sommer I, De Weijer A, Daalman K, Neggers S, Somers M, Kahn R, et al. Can fMRI-guidance improve the efficacy of rTMS treatment for auditory verbal hallucinations? Schizophr Res. 2007;93:406–8.
Kindler J, Homan P, Jann K, Federspiel A, Flury R, Hauf M, et al. Reduced neuronal activity in language-related regions after transcranial magnetic stimulation therapy for auditory verbal hallucinations. Biol Psychiatry. 2013;73:518–24.
Luber BM, Davis S, Bernhardt E, Neacsiu A, Kwapil L, Lisanby SH, et al. Using neuroimaging to individualize TMS treatment for depression: Toward a new paradigm for imaging-guided intervention. Neuroimage. 2017;148:1–7.
Cash RF, Weigand A, Zalesky A, Siddiqi SH, Downar J, Fitzgerald PB, et al. Using brain imaging to improve spatial targeting of transcranial magnetic stimulation for depression. Biol Psychiatry. 2021;90:689–700.
Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72:595–603.
Cash RF, Zalesky A, Thomson RH, Tian Y, Cocchi L, Fitzgerald PB. Subgenual functional connectivity predicts antidepressant treatment response to transcranial magnetic stimulation: independent validation and evaluation of personalization. Biol Psychiatry. 2019;86:e5–e7.
Cash RF, Cocchi L, Lv J, Fitzgerald PB, Zalesky A. Functional magnetic resonance imaging–guided personalization of transcranial magnetic stimulation treatment for depression. JAMA Psychiatry. 2021;78:337–9.
Weigand A, Horn A, Caballero R, Cooke D, Stern AP, Taylor SF, et al. Prospective validation that subgenual connectivity predicts antidepressant efficacy of transcranial magnetic stimulation sites. Biol Psychiatry. 2018;84:28–37.
Ge R, Downar J, Blumberger DM, Daskalakis ZJ, Vila-Rodriguez F. Functional connectivity of the anterior cingulate cortex predicts treatment outcome for rTMS in treatment-resistant depression at 3-month follow-up. Brain Stimul. 2020;13:206–14.
Elbau IG, Lynch CJ, Downar J, Vila-Rodriguez F, Power JD, Solomonov N, et al. Functional connectivity mapping for rTMS target selection in depression. Am J Psychiatry. 2023;180:230–40.
Sangchooli A, Zare-Bidoky M, Jouzdani AF, Schacht J, Bjork JM, Claus ED, et al. Parameter space and potential for biomarker development in 25 years of fMRI drug cue reactivity: a systematic review. JAMA Psychiatry. 2024;81:414–25.
Nummenmaa A, McNab JA, Savadjiev P, Okada Y, Hämäläinen MS, Wang R, et al. Targeting of white matter tracts with transcranial magnetic stimulation. Brain Stimulation. 2014;7:80–4.
De Geeter N, Crevecoeur G, Leemans A, Dupré L. Effective electric fields along realistic DTI-based neural trajectories for modelling the stimulation mechanisms of TMS. Phys Med Biol. 2014;60:453.
Momi D, Ozdemir RA, Tadayon E, Boucher P, Di Domenico A, Fasolo M, et al. Perturbation of resting-state network nodes preferentially propagates to structurally rather than functionally connected regions. Sci Rep. 2021;11:12458.
Wilson S. Commentary: Substance use and the brain: it is not straightforward to differentiate cause from consequence–a commentary on Kim‐Spoon et al.(2020). J Child Psychol Psychiatry. 2021;62:437–40.
Joutsa J, Moussawi K, Siddiqi SH, Abdolahi A, Drew W, Cohen AL, et al. Brain lesions disrupting addiction map to a common human brain circuit. Nat Med. 2022;28:1249–55.
Cagnan H, Pedrosa D, Little S, Pogosyan A, Cheeran B, Aziz T, et al. Stimulating at the right time: phase-specific deep Brain Stimulation. Brain. 2017;140:132–45.
Salehinejad MA, Wischnewski M, Ghanavati E, Mosayebi-Samani M, Kuo M-F, Nitsche MA. Cognitive functions and underlying parameters of human brain physiology are associated with chronotype. Nat Commun. 2021;12:4672.
Zhao Z, Shirinpour S, Tran H, Wischnewski M, Opitz A. Intensity-and frequency-specific effects of transcranial alternating current stimulation are explained by network dynamics. J Neural Eng. 2024;21:026024.
Salehinejad M, Kuo M, Nitsche M. The impact of chronotypes and time of the day on tDCS-induced motor cortex plasticity and cortical excitability. Brain Stimul. 2019;12:421.
Sale MV, Ridding MC, Nordstrom MA. Factors influencing the magnitude and reproducibility of corticomotor excitability changes induced by paired associative stimulation. Exp Brain Res. 2007;181:615–26.
Mielke MM, Miller VM. Improving clinical outcomes through attention to sex and hormones in research. Nat Rev Endocrinol. 2021;17:625–35.
Veldema J. Non-invasive Brain Stimulation and sex/polypeptide hormones in reciprocal interactions: a systematic review. Biomedicines. 2023;11:1981.
Rivas-Grajales AM, Barbour T, Camprodon JA, Kritzer MD. The impact of sex hormones on transcranial magnetic stimulation measures of cortical excitability: A systematic review and considerations for clinical practice. Harv Rev Psychiatry. 2023;31:114–23.
Cuypers K, Marsman A. Transcranial magnetic stimulation and magnetic resonance spectroscopy: Opportunities for a bimodal approach in human neuroscience. Neuroimage. 2021;224:117394.
Michou E, Williams S, Vidyasagar R, Downey D, Mistry S, Edden RA, et al. fMRI and MRS measures of neuroplasticity in the pharyngeal motor cortex. Neuroimage. 2015;117:1–10.
Rudroff T, Workman CD, Fietsam AC, Kamholz J. Response variability in transcranial direct current stimulation: why sex matters. Front Psychiatry. 2020;11:585.
Salehinejad MA, Ghanavati E, Kuo M-F, Nitsche MA. The role of circadian preferred time of day and sleep pressure in tDCS-induced neuroplasticity and associated cognition. Brain Stimul. 2023;16:203–4.
Yu J, Wu Y, Wu B, Xu C, Cai J, Wen X, et al. Sleep patterns correlates with the efficacy of tDCS on post-stroke patients with prolonged disorders of consciousness. J Transl Med. 2022;20:601.
Sun X, Doose J, Faller J, McIntosh JR, Saber GT, Huffman S, et al. Biomarkers predict the efficacy of closed-loop rTMS treatment for refractory depression. Research Square. 2023.
Wischnewski M, Haigh ZJ, Shirinpour S, Alekseichuk I, Opitz A. The phase of sensorimotor mu and beta oscillations has the opposite effect on corticospinal excitability. Brain Stimul. 2022;15:1093–100.
Opitz A, Yeagle E, Thielscher A, Schroeder C, Mehta AD, Milham MP. On the importance of precise electrode placement for targeted transcranial electric stimulation. Neuroimage. 2018;181:560–7.
Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M. Gyri-precise head model of transcranial direct current stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul. 2009;2:201–7. e1.
Soleimani G, Saviz M, Bikson M, Towhidkhah F, Kuplicki R, Paulus MP, et al. Group and individual level variations between symmetric and asymmetric DLPFC montages for tDCS over large scale brain network nodes. Sci Rep. 2021;11:1271.
Soleimani G, Kuplicki R, Camchong J, Opitz A, Paulus MP, Lim KO, et al. Are we really targeting and stimulating DLPFC by placing transcranial electrical stimulation (tES) electrodes over F3/F4? : Wiley Online Library; 2023. Report No.: 1065-9471.
Soleimani G, Kupliki R, Bodurka J, Paulus MP, Ekhtiari H. How structural and functional MRI can inform dual-site tACS parameters: A case study in a clinical population and its pragmatic implications. Brain Stimul. 2022;15:337–51.
McCalley DM, Hanlon CA. Regionally specific gray matter volume is lower in alcohol use disorder: Implications for noninvasive Brain Stimulation treatment. Alcohol Clin Exp Res. 2021;45:1672–83.
Laakso I, Mikkonen M, Koyama S, Hirata A, Tanaka S. Can electric fields explain inter-individual variability in transcranial direct current stimulation of the motor cortex? Sci Rep. 2019;9:626.
Soleimani G, Joutsa J, Moussawi K, Siddiqi SH, Kuplicki R, Bikson M, et al. Converging evidence for frontopolar cortex as a target for neuromodulation in addiction treatment. Am J Psychiatry. 2024;181:100–14.
Soleimani G, Towhidkhah F, Saviz M, Ekhtiari H. Cortical morphology in cannabis use disorder: implications for transcranial direct current stimulation treatment. Basic Clin Neurosci. 2023;14:647.
Bikson M, Esmaeilpour Z, Adair D, Kronberg G, Tyler WJ, Antal A, et al. Transcranial electrical stimulation nomenclature. Brain Stimul. 2019;12:1349–66.
Schwippel T, Pupillo F, Feldman Z, Walker C, Townsend L, Rubinow D, et al. Closed-loop transcranial alternating current stimulation for the treatment of major depressive disorder: an open-label pilot study. Am J Psychiatry. 2024:appi. ajp. 20230838.
Linkovski O, Naftalovich H, David M, Seror Y, Kalanthroff E. The effect of symptom-provocation on inhibitory control in obsessive-compulsive disorder patients is contingent upon chronotype and time of day. J Clin Med. 2023;12:4075.
Tendler A, Sisko E, Gersner R, Garrison S, Mayfield H, Sutton J, et al. Deep TMS for OCD with the H7 coil: community case series. Brain Stimulation. 2019;12:e140.
Sollmann N, Tanigawa N, Bulubas L, Sabih J, Zimmer C, Ringel F, et al. Clinical factors underlying the inter-individual variability of the resting motor threshold in navigated transcranial magnetic stimulation motor mapping. Brain Topogr. 2017;30:98–121.
Oathes DJ, Figueroa Gonzalez A, Grier J, Blaine C, Garcia SD, Linn KJ. Clinical response to fMRI-guided compared to non-image guided rTMS in depression and PTSD: a randomized trial. medRxiv. 2024. 2024.07. 29.24311191
Zrenner C, Ziemann U. Closed-loop Brain Stimulation. Biol Psychiatry. 2024;95:545–52.
Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep Brain Stimulation for treatment-resistant depression. Neuron. 2005;45:651–60.
Holtzheimer PE, Husain MM, Lisanby SH, Taylor SF, Whitworth LA, McClintock S, et al. Subcallosal cingulate deep Brain Stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry. 2017;4:839–49.
Del Mauro L, Vergallito A, Devoto F, Locatelli G, Hassan G, Romero Lauro LJ. Beyond the surface: Deep TMS efficacy in reducing craving in addictive disorders. a systematic review and meta-analysis. medRxiv. 2024:2024.11. 13.24317232.
Li K, Qian L, Zhang C, Li R, Zeng J, Xue C, et al. Deep transcranial magnetic stimulation for treatment-resistant obsessive-compulsive disorder: A meta-analysis of randomized-controlled trials. J Psychiatr Res. 2024;180:96–102.
Bikson M, Inoue M, Akiyama H, Deans JK, Fox JE, Miyakawa H, et al. Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. J Physiol. 2004;557:175–90.
Bijsterbosch JD, Barker AT, Lee K-H, Woodruff PW. Where does transcranial magnetic stimulation (TMS) stimulate? Modelling of induced field maps for some common cortical and cerebellar targets. Med Biol Eng Comput. 2012;50:671–81.
Evans C, Bachmann C, Lee JS, Gregoriou E, Ward N, Bestmann S. Dose-controlled tDCS reduces electric field intensity variability at a cortical target site. Brain Stimulation. 2020;13:125–36.
Cole EJ, Stimpson KH, Bentzley BS, Gulser M, Cherian K, Tischler C, et al. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. Am J Psychiatry. 2020;177:716–26.
Schwippel T, Pupillo F, Feldman Z, Walker C, Townsend L, Rubinow D, et al. Closed-loop transcranial alternating current stimulation for the treatment of major depressive disorder: an open-label pilot study. Am J Psychiatry. 2024;181:842–5.
Morriss R, Briley PM, Webster L, Abdelghani M, Barber S, Bates P, et al. Connectivity-guided intermittent theta burst versus repetitive transcranial magnetic stimulation for treatment-resistant depression: a randomized controlled trial. Nat Med. 2024;30:403–13.
Xiao J, Ming Y, Li L, Huang X, Zhou Y, Ou J, et al. Personalized theta-burst stimulation enhances social skills in young minimally verbal children with autism: a double-blind randomized controlled trial. Biol Psychiatry. 2025. https://doi.org/10.1016/j.biopsych.2025.01.002.
Funding
Research reported in this publication was supported by the University of Minnesota’s MnDRIVE (Minnesota’s Discovery, Research and Innovation Economy) initiative awarded to GS and the University of Minnesota’s Medical Discovery Team on Addiction (MDTA). AO also acknowledges the support of the Minnesota Partnership for Biotechnology & Medical Genomics
Author information
Authors and Affiliations
Contributions
GS developed the idea under the supervision of HE. GS wrote the main manuscript based on inputs from HE, MAN, CH, AO, and KOL. GS developed Figure.
Corresponding authors
Ethics declarations
Competing interests
MAN is in the scientific advisory boards of Neuroelectrics, and Precisis. CH is a member of BrainsWay’s senior leadership (Nov. 2022) and has a financial interest in BrainsWay. She has previously served on an advisory board for Welcony-Magstim and as a consultant for BrainsWay and Roswell Park Cancer Center. AO is an inventor on patents and patent applications describing methods and devices for non-invasive Brain Stimulation. Research reported in this publication was supported by the University of Minnesota’s MnDRIVE (Minnesota’s Discovery, Research and Innovation Economy) initiative awarded to GS and the University of Minnesota’s Medical Discovery Team on Addiction (MDTA). AO also acknowledges the support of the Minnesota Partnership for Biotechnology & Medical Genomics. The remaining authors have nothing to disclose
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The copyright holder for this article was incorrectly given as ‘This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply 2025’ but should have been ‘The Author(s), under exclusive licence to American College of Neuropsychopharmacology 2025’.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Soleimani, G., Nitsche, M.A., Hanlon, C.A. et al. Four dimensions of individualization in brain stimulation for psychiatric disorders: context, target, dose, and timing. Neuropsychopharmacol. 50, 857–870 (2025). https://doi.org/10.1038/s41386-025-02094-3
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-025-02094-3
This article is cited by
-
Optimizing tACS for working memory: differential outcomes in healthy aging and non-amnestic mild cognitive impairment
Alzheimer's Research & Therapy (2025)