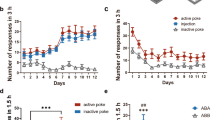
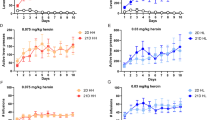
Abstract
Substance use disorder (SUD) has been linked with social impairments. The social cognitive dysfunctions can further increase the risk of the development of SUD or relapse. Therefore, understanding the neural mechanism of substance exposure-associated social impairments is beneficial for the development of novel prevention or treatment strategies for SUD. The prefrontal cortex (PFC) is a key brain region involved in both social cognition and drug addiction. Specifically, the prelimbic part of PFC (PrL) regulates social interaction and heroin-seeking behavior. Therefore, in this study, we explored how PFC excitatory neurons respond to social stimuli after prolonged abstinence from heroin self-administration (SA). Using fiber photometry calcium imaging, we monitored calcium-dependent fluorescent signals in PrL CaMKII-expressing neurons during drug seeking and social interaction tests following 14 days of abstinence from heroin SA. We found that GCaMP6f signals in PrL CaMKII-expressing neurons were increased when heroin-associated cues were presented during drug-seeking tests in both male and female mice after prolonged heroin abstinence, although the baseline neuronal activity in home cage is lower in the heroin group. Conversely, the calcium signals in PrL CaMKII-expressing neurons during social investigation were decreased after heroin abstinence in both sexes, along with reduced total social interaction time. In addition, drug-seeking behavior is partially negatively correlated with social investigation time. These findings provide direct evidence showing that opioid exposure impairs the PFC functional response to social stimuli, which may potentially increase the risk for opioid relapse.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available in the article and in its online supplementary material.
References
Diagnostic and statistical manual of mental disorders: DSM-5™, 5th ed. Arlington, VA, USA: American Psychiatric Publishing, Inc.; 2013.
Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies-tackling the opioid-overdose epidemic. N Engl J Med. 2014;370:2063–6.
Brice-Tutt AC, Wilson LL, Eans SO, Stacy HM, Simons CA, Simpson GG, et al. Multifunctional opioid receptor agonism and antagonism by a novel macrocyclic tetrapeptide prevents reinstatement of morphine-seeking behaviour. Br J Pharmacol. 2020;177:4209–22.
Simpson KJ, Moran MT, Foster ML, Shah DT, Chung DY, Nichols SD, et al. Descriptive, observational study of pharmaceutical and non-pharmaceutical arrests, use, and overdoses in Maine. BMJ Open. 2019;9:e027117.
Dugosh K, Abraham A, Seymour B, McLoyd K, Chalk M, Festinger D. A systematic review on the use of psychosocial interventions in conjunction with medications for the treatment of opioid addiction. J Addict Med. 2016;10:93–103.
Singh A, Xie Y, Davis A, Wang ZJ. Early social isolation stress increases addiction vulnerability to heroin and alters c-Fos expression in the mesocorticolimbic system. Psychopharmacology. 2022;239:1081–95.
Fosnocht AQ, Lucerne KE, Ellis AS, Olimpo NA, Briand LA. Adolescent social isolation increases cocaine seeking in male and female mice. Behav Brain Res. 2019;359:589–96.
Goeldner C, Lutz PE, Darcq E, Halter T, Clesse D, Ouagazzal AM, et al. Impaired emotional-like behavior and serotonergic function during protracted abstinence from chronic morphine. Biol Psychiatry. 2011;69:236–44.
Christie NC. The role of social isolation in opioid addiction. Soc Cogn Affect Neurosci. 2021;16:645–56.
Kadam M, Sinha A, Nimkar S, Matcheswalla Y, De Sousa A. A comparative study of factors associated with relapse in alcohol dependence and opioid dependence. Indian J Psychol Med. 2017;39:627–33.
Smyth BP, Barry J, Keenan E, Ducray K. Lapse and relapse following inpatient treatment of opiate dependence. Ir Med J. 2010;103:176–9.
Koob GF, Le MoalM. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129.
Martin JA, Werner CT, Mitra S, Zhong P, Wang ZJ, Gobira PH, et al. A novel role for the actin-binding protein drebrin in regulating opiate addiction. Nat Commun. 2019;10:4140.
Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–69.
Mazei-Robison MS, Nestler EJ. Opiate-induced molecular and cellular plasticity of ventral tegmental area and locus coeruleus catecholamine neurons. Cold Spring Harb Perspect Med. 2012;2:a012070.
Sklair-Tavron L, Shi WX, Lane SB, Harris HW, Bunney BS, Nestler EJ. Chronic morphine induces visible changes in the morphology of mesolimbic dopamine neurons. Proc Natl Acad Sci USA. 1996;93:11202–7.
Volkow ND, Baler RD, Goldstein RZ. Addiction: pulling at the neural threads of social behaviors. Neuron. 2011;69:599–602.
Grusser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, et al. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology. 2004;175:296–302.
Daglish MR, Weinstein A, Malizia AL, Wilson S, Melichar JK, Britten S, et al. Changes in regional cerebral blood flow elicited by craving memories in abstinent opiate-dependent subjects. Am J Psychiatry. 2001;158:1680–6.
Huhn AS, Sweeney MM, Brooner RK, Kidorf MS, Tompkins DA, Ayaz H, et al. Prefrontal cortex response to drug cues, craving, and current depressive symptoms are associated with treatment outcomes in methadone-maintained patients. Neuropsychopharmacology. 2019;44:826–33.
Zijlstra F, Veltman DJ, Booij J, van den Brink W, Franken IH. Neurobiological substrates of cue-elicited craving and anhedonia in recently abstinent opioid-dependent males. Drug Alcohol Depend. 2009;99:183–92.
Reiner DJ, Fredriksson I, Lofaro OM, Bossert JM, Shaham Y. Relapse to opioid seeking in rat models: behavior, pharmacology and circuits. Neuropsychopharmacology. 2019;44:465–77.
Schmidt ED, Voorn P, Binnekade R, Schoffelmeer AN, De Vries TJ. Differential involvement of the prelimbic cortex and striatum in conditioned heroin and sucrose seeking following long-term extinction. Eur J Neurosci. 2005;22:2347–56.
Bossert JM, Stern AL, Theberge FR, Cifani C, Koya E, Hope BT, et al. Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nat Neurosci. 2011;14:420–2.
Rubio FJ, Quintana-Feliciano R, Warren BL, Li X, Witonsky KFR, Valle FSD, et al. Prelimbic cortex is a common brain area activated during cue-induced reinstatement of cocaine and heroin seeking in a polydrug self-administration rat model. Eur J Neurosci. 2019;49:165–78.
Rogers JL, Ghee S, See RE. The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience. 2008;151:579–88.
Clarke RE, Grant RI, Woods SN, Pagoota BE, Buchmaier S, Bordieanu B, et al. Corticostriatal ensemble dynamics across heroin self-administration to reinstatement. bioRxiv. 2024. https://doi.org/10.1101/2024.06.21.599790.
Kokane SS, Cole RD, Bordieanu B, Ray CM, Haque IA, Otis JM, et al. Increased excitability and synaptic plasticity of Drd1- and Drd2-expressing prelimbic neurons projecting to nucleus accumbens after heroin abstinence are reversed by cue-induced relapse and protein kinase A inhibition. J Neurosci. 2023;43:4019–32.
Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–77.
Lee E, Rhim I, Lee JW, Ghim JW, Lee S, Kim E, et al. Enhanced neuronal activity in the medial prefrontal cortex during social approach behavior. J Neurosci. 2016;36:6926–36.
Yashima J, Uekita T, Sakamoto T. The prelimbic cortex but not the anterior cingulate cortex plays an important role in social recognition and social investigation in mice. PLoS ONE. 2023;18:e0284666.
Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–8.
Liang B, Zhang L, Barbera G, Fang W, Zhang J, Chen X, et al. Distinct and dynamic ON and OFF neural ensembles in the prefrontal cortex code social exploration. Neuron. 2018;100:700–14.e9.
Wang ZJ, Zhong P, Ma K, Seo JS, Yang F, Hu Z, et al. Amelioration of autism-like social deficits by targeting histone methyltransferases EHMT1/2 in Shank3-deficient mice. Mol Psychiatry. 2020;25:2517–33.
Guo B, Chen J, Chen Q, Ren K, Feng D, Mao H, et al. Anterior cingulate cortex dysfunction underlies social deficits in Shank3 mutant mice. Nat Neurosci. 2019;22:1223–34.
Wang YJ, Zan GY, Xu C, Li XP, Shu X, Yao SY, et al. The claustrum-prelimbic cortex circuit through dynorphin/kappa-opioid receptor signaling underlies depression-like behaviors associated with social stress etiology. Nat Commun. 2023;14:7903.
Wang Z-J, Shwani T, Liu J, Zhong P, Yang F, Schatz K, et al. Molecular and cellular mechanisms for differential effects of chronic social isolation stress in males and females. Mol Psychiatry. 2022;27:3056–68.
Tan T, Wang W, Liu T, Zhong P, Conrow-Graham M, Tian X, et al. Neural circuits and activity dynamics underlying sex-specific effects of chronic social isolation stress. Cell Rep. 2021;34:108874.
Gregg L, Barrowclough C, Haddock G. Reasons for increased substance use in psychosis. Clin Psychol Rev. 2007;27:494–510.
Krystal JH, D’Souza DC, Gallinat J, Driesen N, Abi-Dargham A, Petrakis I, et al. The vulnerability to alcohol and substance abuse in individuals diagnosed with schizophrenia. Neurotox Res. 2006;10:235–52.
Smith JP, Book SW. Anxiety and substance use disorders: a review. Psychiatr. 2008;25:19–23.
Anderson EM, Engelhardt A, Demis S, Porath E, Hearing MC. Remifentanil self-administration in mice promotes sex-specific prefrontal cortex dysfunction underlying deficits in cognitive flexibility. Neuropsychopharmacology. 2021;46:1734–45.
Li G, Yue S, Wang Y, Singh A, Wang Z-J. IGF-1 microinjection in the prefrontal cortex attenuates fentanyl-seeking behavior in mice. Int J Neuropsychopharmacol. 2023;26:359–71.
Van den Oever MC, Goriounova NA, Wan Li K, Van der Schors RC, Binnekade R, Schoffelmeer AN, et al. Prefrontal cortex AMPA receptor plasticity is crucial for cue-induced relapse to heroin-seeking. Nat Neurosci. 2008;11:1053–58.
Martin JA, Caccamise A, Werner CT, Viswanathan R, Polanco JJ, Stewart AF, et al. A novel role for oligodendrocyte precursor cells (OPCs) and Sox10 in mediating cellular and behavioral responses to heroin. Neuropsychopharmacology. 2018;43:1385–94.
Wang ZJ, Martin JA, Gancarz AM, Adank DN, Sim FJ, Dietz DM. Activin A is increased in the nucleus accumbens following a cocaine binge. Sci Rep. 2017;7:43658.
Wang ZJ, Martin JA, Mueller LE, Caccamise A, Werner CT, Neve RL, et al. BRG1 in the nucleus accumbens regulates cocaine-seeking behavior. Biol Psychiatry. 2016;80:652–60.
Gancarz AM, Wang ZJ, Schroeder GL, Damez-Werno D, Braunscheidel KM, Mueller LE, et al. Activin receptor signaling regulates cocaine-primed behavioral and morphological plasticity. Nat Neurosci. 2015;18:959–61.
Wang Y, Singh A, Li G, Yue S, Hertel K, Wang Z-J. Opioid induces increased DNA damage in prefrontal cortex and nucleus accumbens. Pharmacol Biochem Behav. 2023;224:173535.
Martin-Garcia E, Burokas A, Kostrzewa E, Gieryk A, Korostynski M, Ziolkowska B, et al. New operant model of reinstatement of food-seeking behavior in mice. Psychopharmacology. 2011;215:49–70.
Soria G, Barbano MF, Maldonado R, Valverde O. A reliable method to study cue-, priming-, and stress-induced reinstatement of cocaine self-administration in mice. Psychopharmacology. 2008;199:593–603.
Wilkerson JL, Ghosh S, Mustafa M, Abdullah RA, Niphakis MJ, Cabrera R, et al. The endocannabinoid hydrolysis inhibitor SA-57: Intrinsic antinociceptive effects, augmented morphine-induced antinociception, and attenuated heroin seeking behavior in mice. Neuropharmacology. 2017;114:156–67.
Shalev U, Morales M, Hope B, Yap J, Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology. 2001;156:98–107.
Kim CK, Yang SJ, Pichamoorthy N, Young NP, Kauvar I, Jennings JH, et al. Simultaneous fast measurement of circuit dynamics at multiple sites across the mammalian brain. Nat Methods. 2016;13:325–8.
Calipari ES, Bagot RC, Purushothaman I, Davidson TJ, Yorgason JT, Pena CJ, et al. In vivo imaging identifies temporal signature of D1 and D2 medium spiny neurons in cocaine reward. Proc Natl Acad Sci USA. 2016;113:2726–31.
Rinker JA, Gioia D, Braunscheidel KM, Wayman WN, Hoffman M, Passarella L, et al. Monitoring neural activity during exposure to drugs of abuse with in vivo fiber photometry. bioRxiv. 2018. https://doi.org/10.1101/487546.
Bicks LK, Koike H, Akbarian S, Morishita H. Prefrontal cortex and social cognition in mouse and man. Front Psychol. 2015;6:166005.
Bardo MT, Neisewander JL, Kelly TH. Individual differences and social influences on the neurobehavioral pharmacology of abused drugs. Pharmacol Rev. 2013;65:255–90.
Miczek KA, Yap JJ, Covington HE 3rd. Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther. 2008;120:102–28.
Nader MA, Banks ML. Environmental modulation of drug taking: Nonhuman primate models of cocaine abuse and PET neuroimaging. Neuropharmacology. 2014;76:510–7.
Venniro M, Zhang M, Caprioli D, Hoots JK, Golden SA, Heins C, et al. Volitional social interaction prevents drug addiction in rat models. Nat Neurosci. 2018;21:1520–29.
Zernig G, Kummer KK, Prast JM. Dyadic social interaction as an alternative reward to cocaine. Front Psychiatry. 2013;4:100.
Fritz M, El Rawas R, Salti A, Klement S, Bardo MT, Kemmler G, et al. Reversal of cocaine-conditioned place preference and mesocorticolimbic Zif268 expression by social interaction in rats. Addict Biol. 2011;16:273–84.
Chen BT, Yau H-J, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, et al. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 2013;496:359–62.
Sudhof TC. Neurotransmitter release: the last millisecond in the life of a synaptic vesicle. Neuron. 2013;80:675–90.
Kavalali ET. The mechanisms and functions of spontaneous neurotransmitter release. Nat Rev Neurosci. 2015;16:5–16.
Sara Y, Bal M, Adachi M, Monteggia LM, Kavalali ET. Use-dependent AMPA receptor block reveals segregation of spontaneous and evoked glutamatergic neurotransmission. J Neurosci. 2011;31:5378–82.
Horvath PM, Piazza MK, Monteggia LM, Kavalali ET. Spontaneous and evoked neurotransmission are partially segregated at inhibitory synapses. Elife. 2020;9;e52852.
Peled ES, Newman ZL, Isacoff EY. Evoked and spontaneous transmission favored by distinct sets of synapses. Curr Biol. 2014;24:484–93.
Wang Y, Fathali H, Mishra D, Olsson T, Keighron JD, Skibicka KP, et al. Counting the number of glutamate molecules in single synaptic vesicles. J Am Chem Soc. 2019;141:17507–11.
Fatt P, Katz B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952;117:109–28.
Postlethwaite M, Hennig MH, Steinert JR, Graham BP, Forsythe ID. Acceleration of AMPA receptor kinetics underlies temperature-dependent changes in synaptic strength at the rat calyx of Held. J Physiol. 2007;579:69–84.
Alabi AA, Tsien RW. Synaptic vesicle pools and dynamics. Cold Spring Harb Perspect Biol. 2012;4:a013680.
Stevens CF, Tsujimoto T. Estimates for the pool size of releasable quanta at a single central synapse and for the time required to refill the pool. Proc Natl Acad Sci USA. 1995;92:846–9.
Rosenmund C, Stevens CF. Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron. 1996;16:1197–207.
Robinson TE, Kolb B. Morphine alters the structure of neurons in the nucleus accumbens and neocortex of rats. Synapse. 1999;33:160–2.
Robinson TE, Gorny G, Savage VR, Kolb B. Widespread but regionally specific effects of experimenter- versus self-administered morphine on dendritic spines in the nucleus accumbens, hippocampus, and neocortex of adult rats. Synapse. 2002;46:271–9.
Ballesteros-Yanez I, Ambrosio E, Benavides-Piccione R, Perez J, Torres I, Miguens M, et al. The effects of morphine self-administration on cortical pyramidal cell structure in addiction-prone Lewis rats. Cereb Cortex. 2007;17:238–49.
Li Y, Wang H, Niu L, Zhou Y. Chronic morphine exposure alters the dendritic morphology of pyramidal neurons in visual cortex of rats. Neurosci Lett. 2007;418:227–31.
Qu L, Wang Y, Li Y, Wang X, Li N, Ge S, et al. Decreased Neuronal Excitability in Medial Prefrontal Cortex during Morphine Withdrawal is associated with enhanced SK channel activity and upregulation of small GTPase Rac1. Theranostics. 2020;10:7369–83.
Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science. 1996;273:1868–71.
Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron. 2007;55:697–711.
Fan Y, Fricker D, Brager DH, Chen X, Lu HC, Chitwood RA, et al. Activity-dependent decrease of excitability in rat hippocampal neurons through increases in I(h). Nat Neurosci. 2005;8:1542–51.
Desai NS, Rutherford LC, Turrigiano GG. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nat Neurosci. 1999;2:515–20.
Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW. Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. J Neurosci. 2010;30:7984–92.
Santini E, Muller RU, Quirk GJ. Consolidation of extinction learning involves transfer from NMDA-independent to NMDA-dependent memory. J Neurosci. 2001;21:9009–17.
Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72.
Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28:6046–53.
Radnikow G, Feldmeyer D. Layer- and cell type-specific modulation of excitatory neuronal activity in the neocortex. Front Neuroanat. 2018;12:1.
Liu J, Dietz K, DeLoyht JM, Pedre X, Kelkar D, Kaur J, et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat Neurosci. 2012;15:1621–3.
Colyn L, Venzala E, Marco S, Perez-Otaño I, Tordera RM. Chronic social defeat stress induces sustained synaptic structural changes in the prefrontal cortex and amygdala. Behav Brain Res. 2019;373:112079.
Rein B, Yan Z, Wang ZJ. Diminished social interaction incentive contributes to social deficits in mouse models of autism spectrum disorder. Genes Brain Behav. 2020;19:e12610.
Qin L, Ma K, Wang ZJ, Hu Z, Matas E, Wei J, et al. Social deficits in Shank3-deficient mouse models of autism are rescued by histone deacetylase (HDAC) inhibition. Nat Neurosci. 2018;21:564–75.
Wang ZJ, Rein B, Zhong P, Williams J, Cao Q, Yang F, et al. Autism risk gene KMT5B deficiency in prefrontal cortex induces synaptic dysfunction and social deficits via alterations of DNA repair and gene transcription. Neuropsychopharmacology. 2021;46:1617–26.
Petrie DJ, Knapp KS, Freet CS, Deneke E, Brick TR, Cleveland HH, et al. Prefrontal cortical response to natural rewards and self-reported anhedonia are associated with greater craving among recently withdrawn patients in residential treatment for opioid use disorder. Brain Res Bull. 2022;190:32–41.
Koya E, Spijker S, Voorn P, Binnekade R, Schmidt ED, Schoffelmeer AN, et al. Enhanced cortical and accumbal molecular reactivity associated with conditioned heroin, but not sucrose‐seeking behaviour. J Neurochem. 2006;98:905–15.
Miller CA, Marshall JF. Altered prelimbic cortex output during cue-elicited drug seeking. J Neurosci. 2004;24:6889–97.
Thibeault KC, Leonard MZ, Kondev V, Emerson SD, Bethi R, Lopez AJ, et al. A cocaine-activated ensemble exerts increased control over behavior while decreasing in size. Biol Psychiatry. 2025;97:590–601.
Paniccia JE, Vollmer KM, Green LM, Grant RI, Winston KT, Buchmaier S, et al. Restoration of a paraventricular thalamo-accumbal behavioral suppression circuit prevents reinstatement of heroin seeking. Neuron. 2024;112:772–85.e9.
Vollmer KM, Green LM, Grant RI, Winston KT, Doncheck EM, Bowen CW, et al. An opioid-gated thalamoaccumbal circuit for the suppression of reward seeking in mice. Nat Commun. 2022;13:6865.
Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2003;168:66–74.
McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–63.
Park W-K, Bari A, Jey A, Anderson S, Spealman R, Rowlett J, et al. Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J Neurosci. 2002;22:2916–25.
See RE. Dopamine D1 receptor antagonism in the prelimbic cortex blocks the reinstatement of heroin-seeking in an animal model of relapse. Int J Neuropsychopharmacol. 2009;12:431–36.
Sun W, Rebec GV. The role of prefrontal cortex D1-like and D2-like receptors in cocaine-seeking behavior in rats. Psychopharmacology. 2005;177:315–23.
Quigley JA, Logsdon MK, Turner CA, Gonzalez IL, Leonardo NB, Becker JB. Sex differences in vulnerability to addiction. Neuropharmacology. 2021;187:108491.
Nicolas C, Zlebnik NE, Farokhnia M, Leggio L, Ikemoto S, Shaham Y. Sex differences in opioid and psychostimulant craving and relapse: a critical review. Pharmacol Rev. 2022;74:119–40.
Smethells J, Greer A, Dougen B, Carroll M. Effects of voluntary exercise and sex on multiply-triggered heroin reinstatement in male and female rats. Psychopharmacology. 2020;237:453–63.
Bakhti-Suroosh A, Towers EB, Lynch WJ. A buprenorphine-validated rat model of opioid use disorder optimized to study sex differences in vulnerability to relapse. Psychopharmacology. 2021;238:1029–46.
Bossert JM, Townsend EA, Altidor LKP, Fredriksson I, Shekara A, Husbands S, et al. Sex differences in the effect of chronic delivery of the buprenorphine analogue BU08028 on heroin relapse and choice in a rat model of opioid maintenance. Br J Pharmacol. 2022;179:227–41.
Venniro M, Zhang M, Shaham Y, Caprioli D. Incubation of methamphetamine but not heroin craving after voluntary abstinence in male and female rats. Neuropsychopharmacology. 2017;42:1126–35.
Lopez AJ, Johnson AR, Euston TJ, Wilson R, Nolan SO, Brady LJ, et al. Cocaine self-administration induces sex-dependent protein expression in the nucleus accumbens. Commun Biol. 2021;4:883.
Urban KR, Geng E, Bhatnagar S, Valentino RJ. Age- and sex-dependent impact of repeated social stress on morphology of rat prefrontal cortex pyramidal neurons. Neurobiol Stress. 2019;10:100165.
Knapp KS, Bunce SC, Brick TR, Deneke E, Cleveland HH. Daily associations among craving, affect, and social interactions in the lives of patients during residential opioid use disorder treatment. Psychol Addict Behav. 2021;35:609–20.
Acknowledgements
We would like to thank the NIDA drug supply program for providing controlled substances. We would like to thank Magnus Marciniak, Christian Brenden Young and Dr. Jai Subramanian for helping with the data analysis.
Funding
ZJW is supported by NIH grants DA050908 and DA056804. YW, SY, and LC are supported by the Sobek-Zhang Foundation. ESC is supported by NIH grant DA048931.
Author information
Authors and Affiliations
Contributions
YW and JL: performed experiments, analyzed data, and drafted the manuscript. SY, LC and AS: performed experiments and analyzed data. TY: analyzed data. ESC: experiment design advising and manuscript preparation. ZJW: designed experiments, supervised the project, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Liu, J., Yue, S. et al. Prefrontal cortex excitatory neurons show distinct response to heroin-associated cue and social stimulus after prolonged heroin abstinence in mice. Neuropsychopharmacol. 50, 1284–1297 (2025). https://doi.org/10.1038/s41386-025-02102-6
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-025-02102-6