Abstract
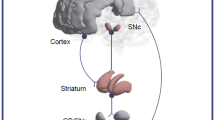
Approximately 30% of patients with schizophrenia do not respond to antipsychotics. While schizophrenia has been primarily explained by the dopamine dysfunction hypothesis, treatment-resistant schizophrenia (TRS) may involve a different pathophysiology. Neuromelanin (NM), a product of dopamine metabolism in the substantia nigra (SN), indirectly measures long-term dopamine synthesis capacity. Few studies have examined SN NM levels in TRS. Therefore, we investigated the relationship between SN NM levels and treatment responsiveness in schizophrenia. We included age- and sex-matched TRS, patients with schizophrenia in remission of positive symptoms (SZ-R), and healthy controls (HCs). Neuromelanin-sensitive magnetic resonance imaging was used to measure SN NM signals. We also evaluated clinical symptoms and cognitive impairment. We conducted voxel-wise analyses of NM contrast-to-noise ratio (CNR) to compare groups pairwise. Correlation analyses examined relationships between NM signals and symptom severity. Seventy-two participants (n = 24 per group) completed the study. The TRS group had higher dorsal SN CNR than the HC group (510 out of 1948 voxels at p < 0.05, corrected p = 0.005, permutation test). In contrast, no significant differences were observed in the other comparisons. No significant correlations were found between NM CNR and clinical severity. Our findings contrast with previous positron emission tomography studies on dorsal striatal dopamine function. Since the dorsal SN contributes to both the mesolimbic and nigrostriatal pathways, with a relatively greater role in the former, dopamine functions in these pathways may play different roles for treatment responsiveness. Further research with multimodal imaging is needed to examine dopamine function and antipsychotic treatment responsiveness in schizophrenia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Kennedy JL, Altar CA, Taylor DL, Degtiar I, Hornberger JC. The social and economic burden of treatment-resistant schizophrenia: a systematic literature review. Int Clin Psychopharmacol. 2014;29:63–76.
Wada M, Noda Y, Iwata Y, Tsugawa S, Yoshida K, Tani H, et al. Dopaminergic dysfunction and excitatory/inhibitory imbalance in treatment-resistant schizophrenia and novel neuromodulatory treatment. Mol Psychiatry. 2022;27:2950–67.
Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci USA. 2000;97:8104–9.
Caravaggio F, Borlido C, Wilson A, Graff-Guerrero A. Examining endogenous dopamine in treated schizophrenia using [11C]-(+)-PHNO positron emission tomography: A pilot study. Clin Chim Acta. 2015;449:60–2.
Howes OD, Montgomery AJ, Asselin M-C, Murray RM, Grasby PM, McGuire PK. Molecular imaging studies of the striatal dopaminergic system in psychosis and predictions for the prodromal phase of psychosis. Br J Psychiatry. 2007;51:s13–8.
McCutcheon R, Beck K, Jauhar S, Howes OD. Defining the locus of dopaminergic dysfunction in schizophrenia: A meta-analysis and test of the mesolimbic hypothesis. Schizophr Bull. 2018;44:1301–11.
Jauhar S, Veronese M, Nour MM, Rogdaki M, Hathway P, Turkheimer FE, et al. Determinants of treatment response in first-episode psychosis: an 18F-DOPA PET study. Mol Psychiatry. 2019;24:1502–12.
Kim E, Howes OD, Veronese M, Beck K, Seo S, Park JW, et al. Presynaptic dopamine capacity in patients with treatment-resistant schizophrenia taking clozapine: An [18F]DOPA PET study. Neuropsychopharmacology. 2017;42:941–50.
Demjaha A, Murray RM, McGuire PK, Kapur S, Howes OD. Dopamine synthesis capacity in patients with treatment-resistant schizophrenia. Am J Psychiatry. 2012;169:1203–10.
Egerton A, Murphy A, Donocik J, Anton A, Barker GJ, Collier T, et al. Dopamine and Glutamate in Antipsychotic-Responsive Compared With Antipsychotic-Nonresponsive Psychosis: A Multicenter Positron Emission Tomography and Magnetic Resonance Spectroscopy Study (STRATA). Schizophr Bull. 2020;47:505–16.
Avram M, Brandl F, Cabello J, Leucht C, Scherr M, Mustafa M, et al. Reduced striatal dopamine synthesis capacity in patients with schizophrenia during remission of positive symptoms. Brain. 2019;142:1813–26.
Demjaha A, Egerton A, Murray RM, Kapur S, Howes OD, Stone JM, et al. Antipsychotic treatment resistance in schizophrenia associated with elevated glutamate levels but normal dopamine function. Biol Psychiatry. 2014;75:e11–3.
Cassidy CM, Zucca FA, Girgis RR, Baker SC, Weinstein JJ, Sharp ME, et al. Neuromelanin-sensitive MRI as a noninvasive proxy measure of dopamine function in the human brain. Proc Natl Acad Sci. 2019;116:5108–17.
Ueno F, Iwata Y, Nakajima S, Caravaggio F, Rubio JM, Horga G, et al. Neuromelanin accumulation in patients with schizophrenia: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2022;132:1205–13.
van der Pluijm M, Wengler K, Reijers PN, Cassidy CM, Tjong Tjin Joe K, de Peuter OR, et al. Neuromelanin-Sensitive MRI as Candidate Marker for Treatment Resistance in First-Episode Schizophrenia. Am J Psychiatry. 2024;181:512–9.
Wengler K, Baker SC, Velikovskaya A, Fogelson A, Girgis RR, Reyes-Madrigal F, et al. Generalizability and out-of-sample predictive ability of associations between neuromelanin-sensitive magnetic resonance imaging and psychosis in antipsychotic-free individuals. JAMA Psychiatry. 2024;81:198–208.
Conn K-A, Burne THJ, Kesby JP. Subcortical dopamine and cognition in schizophrenia: Looking beyond psychosis in preclinical models. Front Neurosci. 2020;14:542.
Slifstein M, van de Giessen E, Van Snellenberg J, Thompson JL, Narendran R, Gil R, et al. Deficits in prefrontal cortical and extrastriatal dopamine release in schizophrenia: a positron emission tomographic functional magnetic resonance imaging study. JAMA Psychiatry. 2015;72:316–24.
Arbanas G. Diagnostic and statistical manual of mental disorders (DSM-5). Alcoholism Psychiatry Res. 2015;51:61–4.
Howes OD, McCutcheon R, Agid O, de Bartolomeis A, van Beveren NJM, Birnbaum ML, et al. Treatment-Resistant Schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) Working Group Consensus Guidelines on Diagnosis and Terminology. Am J Psychiatry. 2017;174:216–29.
Iwata Y, Nakajima S, Plitman E, Caravaggio F, Kim J, Shah P, et al. Glutamatergic Neurometabolite Levels in Patients With Ultra-Treatment-Resistant Schizophrenia: A Cross-Sectional 3T Proton Magnetic Resonance Spectroscopy Study. Biol Psychiatry. 2019;85:596–605.
Andreasen NC, Carpenter WT Jr, Kane JM, Lasser RA, Marder SR, Weinberger DR. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry. 2005;162:441–9.
Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76.
Guy W ECDEU assessment manual for psychopharmacology. U.S. Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976.
Jones SH, Thornicroft G, Coffey M, Dunn G. A brief mental health outcome scale-reliability and validity of the Global Assessment of Functioning (GAF). Br J Psychiatry. 1995;166:654–9.
Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20:310–9.
Matsui M, Kasai Y, Nagasaki M. Reliability and validity for the Japanese version of the repeatable battery for the assessment of neuropsychological status (RBANS). Toyama Med J. 2010;21:31–6.
Royall DR, Mahurin RK, Gray KF. Bedside assessment of executive cognitive impairment: the executive interview. J Am Geriatr Soc. 1992;40:1221–6.
Matsuoka T, Kato Y, Taniguchi S, Ogawa M, Fujimoto H, Okamura A, et al. Japanese versions of the executive interview (J-EXIT25) and the executive clock drawing task (J-CLOX) for older people. Int Psychogeriatr. 2014;26:1387–97.
Matsuoka K, Uno M, Kasai K, Koyama K, Kim Y. Estimation of premorbid IQ in individuals with Alzheimer’s disease using Japanese ideographic script (Kanji) compound words: Japanese version of National Adult Reading Test. Psychiatry Clin Neurosci. 2006;60:332–9.
Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12:26–41.
Wengler K, He X, Abi-Dargham A, Horga G. Reproducibility assessment of neuromelanin-sensitive magnetic resonance imaging protocols for region-of-interest and voxelwise analyses. Neuroimage. 2020;208:116457.
Zecca L, Fariello R, Riederer P, Sulzer D, Gatti A, Tampellini D. The absolute concentration of nigral neuromelanin, assayed by a new sensitive method, increases throughout the life and is dramatically decreased in Parkinson’s disease. FEBS Lett. 2002;510:216–20.
Myran DT, Pugliese M, Harrison LD, Solmi M, Anderson KK, Fiedorowicz JG, et al. Changes in incident schizophrenia diagnoses associated with cannabis use disorder after cannabis legalization. JAMA Netw Open. 2025;8:e2457868.
Bloomfield MAP, Ashok AH, Volkow ND, Howes OD. The effects of Δ9-tetrahydrocannabinol on the dopamine system. Nature. 2016;539:369–77.
Ahrens J, Ford SD, Schaefer B, Reese D, Khan AR, Tibbo P, et al. Convergence of cannabis and psychosis on the dopamine system. JAMA Psychiatry. 2025;82:609–17.
Perlman G, Wengler K, Moeller SJ, Kotov R, Klein DN, Weinstein JJ, et al. Association of neuromelanin-sensitive MRI signal with lifetime substance use in young women. Am J Psychiatry. 2024;181:997–1005.
Zucca FA, Vanna R, Cupaioli FA, Bellei C, De Palma A, Di Silvestre D, et al. Neuromelanin organelles are specialized autolysosomes that accumulate undegraded proteins and lipids in aging human brain and are likely involved in Parkinson’s disease. NPJ Parkinsons Dis. 2018;4:17.
Graham DG. On the origin and significance of neuromelanin. Arch Pathol Lab Med. 1979;103:359–62.
Kumakura Y, Cumming P. PET studies of cerebral levodopa metabolism: a review of clinical findings and modeling approaches. Neuroscientist. 2009;15:635–50.
Vano LJ, McCutcheon RA, Rutigliano G, Kaar SJ, Finelli V, Nordio G, et al. Mesostriatal Dopaminergic Circuit Dysfunction in Schizophrenia: A Multimodal Neuromelanin-sensitive MRI and [18F]-DOPA PET Study. Biol Psychiatry. 2024;96:674–683.
van Hooijdonk CFM, van der Pluijm M, Smith C, Yaqub M, van Velden FHP, Horga G, et al. Striatal dopamine synthesis capacity and neuromelanin in the substantia nigra: A multimodal imaging study in schizophrenia and healthy controls. Neuroscience Appl. 2023;2:101134.
Jeste DV, Maglione JE. Treating older adults with schizophrenia: challenges and opportunities. Schizophr Bull. 2013;39:966–8.
Jeste DV, Wolkowitz OM, Palmer BW. Divergent trajectories of physical, cognitive, and psychosocial aging in schizophrenia. Schizophr Bull. 2011;37:451–5.
Nakajima S, Uchida H, Bies RR, Caravaggio F, Suzuki T, Plitman E, et al. Dopamine D2/3 receptor occupancy following dose reduction is predictable with minimal plasma antipsychotic concentrations: An open-label clinical trial. Schizophr Bull. 2016;42:212–9.
Nakajima S, Caravaggio F, Boileau I, Chung JK, Plitman E, Gerretsen P, et al. Lack of age-dependent decrease in dopamine D3 receptor availability: a [(11)C]-(+)-PHNO and [(11)C]-raclopride positron emission tomography study. J Cereb Blood Flow Metab. 2015;35:1812–8.
Graff-Guerrero A, Rajji TK, Mulsant BH, Nakajima S, Caravaggio F, Suzuki T, et al. Evaluation of antipsychotic dose reduction in late-life schizophrenia: A prospective dopamine D2/3 receptor occupancy study: A prospective dopamine D2/3Receptor occupancy study. JAMA Psychiatry. 2015;72:927–34.
Weidenauer A, Sauerzopf U, Bauer M, Bum C, Diendorfer C, Dajic I, et al. Amphetamine-induced dopamine release predicts 1-year outcome in first-episode psychosis: A naturalistic observation. Schizophr Bull. 2024;51:159–69.
Sigvard AK, Nielsen MØ, Gjedde A, Bojesen KB, Fuglø D, Tangmose K, et al. Dopaminergic activity in antipsychotic-naïve patients assessed with positron emission tomography before and after partial dopamine D2 receptor agonist treatment: Association with psychotic symptoms and treatment response. Biol Psychiatry. 2022;91:236–45.
Brugger SP, Angelescu I, Abi-Dargham A, Mizrahi R, Shahrezaei V, Howes OD. Heterogeneity of Striatal Dopamine Function in Schizophrenia: Meta-analysis of Variance. Biol Psychiatry. 2020;87:215–24.
Haber SN. The place of dopamine in the cortico-basal ganglia circuit. Neuroscience. 2014;282:248–57.
Matsuda W, Furuta T, Nakamura KC, Hioki H, Fujiyama F, Arai R, et al. Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J Neurosci. 2009;29:444–53.
Prensa L, Giménez-Amaya JM, Parent A, Bernácer J, Cebrián C The nigrostriatal pathway: axonal collateralization and compartmental specificity. J Neural Transm Suppl. 2009;73:49–58.
Nakajima S, Gerretsen P, Takeuchi H, Caravaggio F, Chow T, Le Foll B, et al. The potential role of dopamine D₃ receptor neurotransmission in cognition. Eur Neuropsychopharmacol. 2013;23:799–813.
Lakhani DA, Zhou X, Tao S, Patel V, Wen S, Okromelidze L, et al. Diagnostic utility of 7T neuromelanin imaging of the substantia nigra in Parkinson’s disease. NPJ Parkinsons Dis. 2024;10:13.
van Hooijdonk CFM, Drukker M, van de Giessen E, Booij J, Selten J-P, van Amelsvoort TAMJ. Dopaminergic alterations in populations at increased risk for psychosis: A systematic review of imaging findings. Prog Neurobiol. 2022;213:102265.
Huttunen J, Heinimaa M, Svirskis T, Nyman M, Kajander J, Forsback S, et al. Striatal dopamine synthesis in first-degree relatives of patients with schizophrenia. Biol Psychiatry. 2008;63:114–7.
Acknowledgements
This study was supported by the Japan Society for the Promotion of Science (18H02755, 22H03002), Japan Agency for Medical Research and Development (AMED: JP24wm0625302), Japan Research Foundation for Clinical Pharmacology, Naito Foundation, Watanabe Foundation and Takeda Science Foundation. We thank Mr. Nishikata for his technical support. We thank the participants and their families for their cooperation in this research.
Funding
SH has received the JSPS Research Fellowship for Young Scientists (DC1), and The Keio University Doctorate Student Grant-in-Aid Program from Ushioda Memorial Fund. FU has received grants from Discovery Fund, Nakatani Foundation, Canadian Institutes of Health Research (CIHR), and Brain & Behavior Research Foundation (BBRF); manuscript fees from Dainippon Sumitomo Pharma; and consultant fees from VeraSci, and Uchiyama Underwriting within the past three years. SN has received grants from Japan Society for the Promotion of Science (18H02755, 22H03002), Japan Agency for Medical Research and development (AMED: JP24wm0625302, JP24wm0625307), Japan Research Foundation for Clinical Pharmacology, Naito Foundation, Takeda Science Foundation, Watanabe Foundation, Osakeno-Kagaku Foundation, and Astellas Foundation within the past three years. SN has also received research support, manuscript fees or speaker’s honoraria from Asahi Quality & Innovations, Ltd., Teijin Pharma, Sumitomo Pharma, Meiji Seika Pharma, Otsuka, PDR pharma, and MSD within the past three years. YN has received Grants-in-Aid for Scientific Research (B) (18H02755; 20H04092; 21H02813) from the Japan Society for the Promotion of Science (JSPS), research grants (a) from Japan Agency for Medical Research and Development (AMED), investigator-initiated clinical study grants from TEIJIN PHARMA LIMITED (Tokyo, Japan) and Inter Reha Co., Ltd. (Tokyo, Japan). YN also receives research grants from Japan Health Foundation, Meiji Yasuda Mental Health Foundation, Mitsui Life Social Welfare Foundation, Takeda Science Foundation, SENSHIN Medical Research Foundation, Health Science Center Foundation, Mochida Memorial Foundation for Medical and Pharmaceutical Research, Taiju Life Social Welfare Foundation, and Daiichi Sankyo Scholarship Donation Program. YN has received speaker’s honoraria from Dainippon Sumitomo Pharma, MOCHIDA PHARMACEUTICAL CO., LTD. (Tokyo, Japan), and Yoshito-miyakuhin Corporation within the past three years. AG-G received the grants from Canadian Institutes of Health Research (S). AG-G also receives research grants from Ministry of Economic Development and Innovation Ontario, Ontario Mental Health Foundation Type A grant, and NARSAD Independent Investigator (AG-G). Other authors do not have any conflict of interest to declare.
Author information
Authors and Affiliations
Contributions
Ryosuke Tarumi: Conceptualization; Investigation; Resources; Data Curation; Writing – Original Draft; Supervision; Project Administration. Shiori Honda: Conceptualization; Methodology; Formal Analysis; Investigation; Data Curation; Writing – Original Draft; Visualization; Supervision. Clifford Cassidy: Methodology; Software; Validation; Writing – Review & Editing; Visualization. Takahide Etani: Validation; Formal Analysis; Data Curation; Writing – Original Draft; Visualization. Saki Homma: Validation; Formal Analysis; Investigation; Data Curation; Writing – Original Draft; Visualization. Shunya Sekihara: Formal Analysis; Data Curation; Writing – Original Draft; Visualization. Yuka Kaneko: Data Curation; Writing – Review & Editing. Sotaro Moriyama: Investigation; Data Curation; Writing – Review & Editing. Yui Tobari: Investigation; Data Curation; Writing – Review & Editing. Koki Takahashi: Validation; Data Curation; Writing – Review & Editing. Fumihiko Ueno: Writing – Review & Editing. Guillermo Horga: Writing – Review & Editing. Sakiko Tsugawa: Validation; Writing – Review & Editing. Jose M. Rubio: Writing – Review & Editing. Mie Matsui: Writing – Review & Editing. Shinya Fujii: Writing – Review & Editing. Ariel Graff-Guerrero: Writing – Review & Editing. Yoshihiro Noda: Conceptualization; Resources; Writing – Review & Editing. Hiroyuki Uchida: Conceptualization; Resources; Writing – Review & Editing; Supervision. Shinichiro Nakajima: Conceptualization; Resources; Writing – Original Draft; Supervision; Project Administration; Funding Acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors have nothing to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tarumi, R., Honda, S., Cassidy, C. et al. Associations of neuromelanin in the substantia nigra with antipsychotic response in schizophrenia. Neuropsychopharmacol. 51, 682–690 (2026). https://doi.org/10.1038/s41386-025-02275-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-025-02275-0