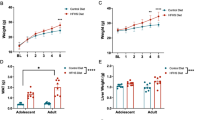
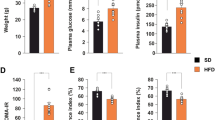
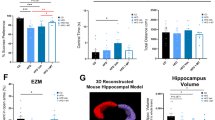
Abstract
Alterations to perineuronal nets (PNNs) are linked to cognitive deficits observed in multiple neuropsychiatric and neurodegenerative conditions. PNNs are proposed both to limit neuronal plasticity and to protect neuronal populations critical for cognitive function. Here, we assessed these two distinct proposed functions of PNNs in the same experimental procedure, varying only the timing of PNN manipulation. Enzymatic degradation of hippocampal PNNs 4 weeks after an obesogenic high-fat high-sugar (HFHS) diet – which causes hippocampal-dependent memory impairments – restored memory performance, demonstrating a key role of PNNs in plasticity. In contrast, degradation of hippocampal PNNs prior to HFHS diet exacerbated memory deficits, demonstrating PNNs’ role in neuroprotection. Because PNNs primarily surround PV+ neurons (PVNs), we hypothesized that the mechanism underlying these effects of PNN degradation would involve, at least in part, alterations in hippocampal PVN function. Thus, to see whether PNN degradation alters the function of hippocampal PVNs, we took fiber photometry measurements across multi-day timescales and found that hippocampal PNN degradation alters calcium events in PVNs. We then went on to explicitly manipulate PVNs and found that chemogenetic inhibition of hippocampal PVNs in mice on a standard diet impaired memory performance, and activation of hippocampal PVNs in mice on a HFHS diet restored memory performance, bidirectional effects that mirror the bidirectional effects of PNN degradation. Taken together, these findings support the dual plasticity and protection hypothesis of PNN function, provide evidence that key mechanisms of memory involve the interaction of PNNs and PVNs, and point to PNNs as a potential therapeutic target in cognitive decline.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Experimental data from these studies are available at https://osf.io/ujg7w/?view_only=10f8d31b8ede45c39d447ca4dd582a3f.
References
Reichelt AC, Hare DJ, Bussey TJ, Saksida LM. Perineuronal nets: plasticity, protection, and therapeutic potential. Trends Neurosci. 2019;42:458–70.
Rossier J, Bernard A, Cabungcal JH, Perrenoud Q, Savoye A, Gallopin T, et al. Cortical fast-spiking parvalbumin interneurons enwrapped in the perineuronal net express the metallopeptidases Adamts8, Adamts15 and Neprilysin. Mol Psychiatry. 2015. https://doi.org/10.1038/mp.2014.162.
Härtig W, Brauer K, Brückner G. Wisteria floribunda agglutinin-labelled nets surround parvalbumin-containing neurons. Neuroreport. 1992. https://doi.org/10.1097/00001756-199210000-00012.
Dityatev A, Schachner M, Sonderegger P. The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat Rev Neurosci. 2010;11:735–46.
Hijazi S, Heistek TS, Scheltens P, Neumann U, Shimshek DR, Mansvelder HD, et al. Early restoration of parvalbumin interneuron activity prevents memory loss and network hyperexcitability in a mouse model of Alzheimer’s disease. Mol Psychiatry. 2019. https://doi.org/10.1038/s41380-019-0483-4.
Donato F, Rompani SB, Caroni P. Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature. 2013. https://doi.org/10.1038/nature12866.
Murray AJ, Woloszynowska-Fraser MU, Ansel-Bollepalli L, Cole KLH, Foggetti A, Crouch B, et al. Parvalbumin-positive interneurons of the prefrontal cortex support working memory and cognitive flexibility. Sci Rep. 2015. https://doi.org/10.1038/srep16778.
Murray AJ, Sauer JF, Riedel G, McClure C, Ansel L, Cheyne L, et al. Parvalbumin-positive CA1 interneurons are required for spatial working but not for reference memory. Nat Neurosci. 2011. https://doi.org/10.1038/nn.2751.
Kim J, Bae JS. Distinct roles of parvalbumin- and somatostatin-expressing interneurons in working memory. Neuron. 2016;2016:6058147 https://doi.org/10.1016/j.neuron.2016.09.023.
Verret L, Mann EO, Hang GB, Barth AMI, Cobos I, Ho K, et al. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in alzheimer model. Cell. 2012. https://doi.org/10.1016/j.cell.2012.02.046.
Gonzalez-Burgos G, Cho RY, Lewis DA. Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol Psychiatry. 2015;77:1031–40.
Glausier JR, Fish KN, Lewis DA. Altered parvalbumin basket cell inputs in the dorsolateral prefrontal cortex of schizophrenia subjects. Mol Psychiatry. 2014. https://doi.org/10.1038/mp.2013.152.
Hashemi E, Ariza J, Rogers H, Noctor SC, Martínez-Cerdeño V. The number of parvalbumin-expressing interneurons is decreased in the medial prefrontal cortex in autism. Cereb Cortex. 2017. https://doi.org/10.1093/cercor/bhw021.
Enwright JF, Sanapala S, Foglio A, Berry R, Fish KN, Lewis DA. Reduced labeling of parvalbumin neurons and perineuronal nets in the dorsolateral prefrontal cortex of subjects with schizophrenia. Neuropsychopharmacology. 2016. https://doi.org/10.1038/npp.2016.24.
Fawcett JW, Oohashi T, Pizzorusso T. The roles of perineuronal nets and the perinodal extracellular matrix in neuronal function. Nat Rev Neurosci. 2019;20:451–65.
Foscarin S, Raha-Chowdhury R, Fawcett JW, Kwok JCF. Brain ageing changes proteoglycan sulfation, rendering perineuronal nets more inhibitory. Aging. 2017. https://doi.org/10.18632/aging.101256.
Romberg C, Yang S, Melani R, Andrews MR, Horner AE, Spillantini MG, et al. Depletion of perineuronal nets enhances recognition memory and long-term depression in the perirhinal cortex. J Neurosci. 2013. https://doi.org/10.1523/JNEUROSCI.6267-11.2013.
Yang S, Cacquevel M, Saksida LM, Bussey TJ, Schneider BL, Aebischer P, et al. Perineuronal net digestion with chondroitinase restores memory in mice with tau pathology. Exp Neurol. 2015. https://doi.org/10.1016/j.expneurol.2014.11.013.
Suttkus A, Rohn S, Jäger C, Arendt T, Morawski M. Neuroprotection against iron-induced cell death by perineuronal nets - An in vivo analysis of oxidative stress. Am J Neurodegener Dis. 2012;1:122–9.
Suttkus A, Holzer M, Morawski M, Arendt T. The neuronal extracellular matrix restricts distribution and internalization of aggregated Tau-protein. Neuroscience. 2016. https://doi.org/10.1016/j.neuroscience.2015.11.040.
Cabungcal JH, Steullet P, Morishita H, Kraftsik R, Cuenod M, Hensch TK, et al. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc Natl Acad Sci USA. 2013. https://doi.org/10.1073/pnas.1300454110.
Morishita H, Cabungcal HJ, Chen Y, Do KQ, Hensch TK. Prolonged period of cortical plasticity upon redox dysregulation in fast-spiking interneurons. Biological Psychiatry. 2012;78:392–402.
Attuquayefio T, Stevenson RJ, Oaten MJ, Francis HM. A four-day Western-style dietary intervention causes reductions in hippocampal-dependent learning and memory and interoceptive sensitivity. PLoS One. 2017. https://doi.org/10.1371/journal.pone.0172645.
McLean FH, Grant C, Morris AC, Horgan GW, Polanski AJ, Allan K, et al. Rapid and reversible impairment of episodic memory by a high-fat diet in mice. Sci Rep. 2018. https://doi.org/10.1038/s41598-018-30265-4.
Baker KD, Reichelt AC. Impaired fear extinction retention and increased anxiety-like behaviours induced by limited daily access to a high-fat/high-sugar diet in male rats: Implications for diet-induced prefrontal cortex dysregulation. Neurobiol Learn Mem. 2016;136:127–38.
Boitard C, Cavaroc A, Sauvant J, Aubert A, Castanon N, Layé S, et al. Impairment of hippocampal-dependent memory induced by juvenile high-fat diet intake is associated with enhanced hippocampal inflammation in rats. Brain Behav Immun. 2014. https://doi.org/10.1016/j.bbi.2014.03.005.
Dingess PM, Harkness JH, Slaker M, Zhang Z, Wulff SS, Sorg BA, et al. Consumption of a high-fat diet alters perineuronal nets in the prefrontal cortex. Neural Plast. 2018;2018:1–8. https://doi.org/10.1155/2018/2108373.
Dingess PM, Zhang Z, Sorg BA, Ferrario CR, Brown TE. Sex and region-specific effects of high fat diet on PNNs in obesity susceptible rats. Physiol Behav. 2020. https://doi.org/10.1016/j.physbeh.2020.112963.
Reichelt AC, Gibson GD, Abbott KN, Hare DJ. A high-fat high-sugar diet in adolescent rats impairs social memory and alters chemical markers characteristic of atypical neuroplasticity and parvalbumin interneuron depletion in the medial prefrontal cortex. Food Funct. 2019;10:1985–98.
Reichelt AC, Lemieux CA, Princz-Lebel O, Singh A, Bussey TJ, Saksida LM. Age-dependent and region-specific alteration of parvalbumin neurons, perineuronal nets and microglia in the mouse prefrontal cortex and hippocampus following obesogenic diet consumption. Sci Rep. 2021;11:5593.
Pintana H, Lietzau G, Lovise Augestad I, Chiazza F, Nyström T, Patrone C, et al. Obesity-induced type 2 diabetes impairs neurological recovery after stroke in correlation with decreased neurogenesis and persistent atrophy of parvalbumin-positive interneurons. Clin Sci. 2019. https://doi.org/10.1042/CS20190180.
Reichelt AC, Killcross S, Hambly LD, Morris MJ, Westbrook RF. Impact of adolescent sucrose access on cognitive control, recognition memory, and parvalbumin immunoreactivity. Learn Mem. 2015;22:215–24.
Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Investig. 2012. https://doi.org/10.1172/JCI59660.
Crapser JD, Spangenberg EE, Barahona RA, Arreola MA, Hohsfield LA, Green KN. Microglia facilitate loss of perineuronal nets in the Alzheimer’s disease brain. EBioMedicine. 2020;58:102919.
Reichelt AC, Morris MJ, Westbrook RF. Daily access to sucrose impairs aspects of spatial memory tasks reliant on pattern separation and neural proliferation in rats. Learn Mem. 2016;23:386–90.
Beilharz JE, Maniam J, Morris MJ. Short-term exposure to a diet high in fat and sugar, or liquid sugar, selectively impairs hippocampal-dependent memory, with differential impacts on inflammation. Behav Brain Res. 2016. https://doi.org/10.1016/j.bbr.2016.03.018.
Bekinschtein P, Kent BA, Oomen CA, Clemenson GD, Gage FH, Saksida LM, et al. Brain-derived neurotrophic factor interacts with adult-born immature cells in the dentate gyrus during consolidation of overlapping memories. Hippocampus. 2014. https://doi.org/10.1002/hipo.22304.
Bekinschtein P, Kent BA, Oomen CA, Clemenson GD, Gage FH, Saksida LM, et al. BDNF in the dentate gyrus is required for consolidation of ‘pattern-separated’ memories. Cell Rep. 2013. https://doi.org/10.1016/j.celrep.2013.09.027.
Kent BA, Beynon AL, Hornsby AKE, Bekinschtein P, Bussey TJ, Davies JS, et al. The orexigenic hormone acyl-ghrelin increases adult hippocampal neurogenesis and enhances pattern separation. Psychoneuroendocrinology. 2015. https://doi.org/10.1016/j.psyneuen.2014.10.015.
Reichelt AC, Kramar CP, Ghosh-Swaby OR, Sheppard PAS, Kent BA, Bekinschtein P, et al. The spontaneous location recognition task for assessing spatial pattern separation and memory across a delay in rats and mice. Nat Protoc. 2021;16:5616–33.
Skirzewski M, Princz-Lebel O, German-Castelan L, Crooks AM, Kim GK, Tarnow SH, et al. Continuous cholinergic-dopaminergic updating in the nucleus accumbens underlies approaches to reward-predicting cues. Nat Commun. 2022;13:7924.
Baufeld C, Osterloh A, Prokop S, Miller KR, Heppner FL. High-fat diet-induced brain region-specific phenotypic spectrum of CNS resident microglia. Acta Neuropathol. 2016. https://doi.org/10.1007/s00401-016-1595-4.
Hainmueller T, Cazala A, Huang LW, Bartos M. Subfield-specific interneuron circuits govern the hippocampal response to novelty in male mice. Nat Commun. 2024;15:714.
Yamada J, Jinno S. Spatio-temporal differences in perineuronal net expression in the mouse hippocampus, with reference to parvalbumin. Neuroscience. 2013. https://doi.org/10.1016/j.neuroscience.2013.08.061.
Erion JR, Wosiski-Kuhn M, Dey A, Hao S, Davis CL, Pollock NK, et al. Obesity elicits interleukin 1-mediated deficits in hippocampal synaptic plasticity. J Neurosci. 2014. https://doi.org/10.1523/JNEUROSCI.4200-13.2014.
Hao S, Dey A, Yu X, Stranahan AM. Dietary obesity reversibly induces synaptic stripping by microglia and impairs hippocampal plasticity. Brain Behav Immun. 2016. https://doi.org/10.1016/j.bbi.2015.08.023.
Yang Y, Duan C, Huang L, Xia X, Zhong Z, Wang B, et al. Juvenile high–fat diet–induced senescent glial cells in the medial prefrontal cortex drives neuropsychiatric behavioral abnormalities in mice. Behav Brain Res. 2020;395:112838.
Végh MJ, Heldring CM, Kamphuis W, Hijazi S, Timmerman AJ, Li KW, et al. Reducing hippocampal extracellular matrix reverses early memory deficits in a mouse model of Alzheimer’s disease. Acta Neuropathol Commun. 2014. 2014. https://doi.org/10.1186/s40478-014-0076-z.
Carulli D, Pizzorusso T, Kwok JCF, Putignano E, Poli A, Forostyak S, et al. Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain. 2010. https://doi.org/10.1093/brain/awq145.
Inan M, Zhao M, Manuszak M, Karakaya C, Rajadhyaksha AM, Pickel VM, et al. Energy deficit in parvalbumin neurons leads to circuit dysfunction, impaired sensory gating and social disability. Neurobiol Dis. 2016. https://doi.org/10.1016/j.nbd.2016.04.004.
Harland M, Torres S, Liu J, Wang X. Neuronal mitochondria modulation of LPS-induced neuroinflammation. J Neurosci. 2020. https://doi.org/10.1523/JNEUROSCI.2324-19.2020.
Aguilar-Valles A, Inoue W, Rummel C, Luheshi GN. Obesity, adipokines and neuroinflammation. Neuropharmacology. 2015;96:124–34.
Buckman LB, Hasty AH, Flaherty DK, Buckman CT, Thompson MM, Matlock BK, et al. Obesity induced by a high-fat diet is associated with increased immune cell entry into the central nervous system. Brain Behav Immun. 2014;35:33–42.
Dwir D, Giangreco B, Xin L, Tenenbaum L, Cabungcal JH, Steullet P, et al. MMP9/RAGE pathway overactivation mediates redox dysregulation and neuroinflammation, leading to inhibitory/excitatory imbalance: a reverse translation study in schizophrenia patients. Mol Psychiatry. 2019. https://doi.org/10.1038/s41380-019-0393-5.
Kettenmann H, Kirchhoff F, Verkhratsky A. Microglia: new roles for the synaptic stripper. Neuron. 2013;77:10–18.
Salter MW, Stevens B. Microglia emerge as central players in brain disease. Nat Med. 2017;23:1018–27.
Beddows CA, Shi F, Horton AL, Dalal S, Zhang P, Ling C-C, et al. Pathogenic hypothalamic extracellular matrix promotes metabolic disease. Nature. 2024;633:914–22.
Bjerke IE, Yates SC, Laja A, Witter MP, Puchades MA, Bjaalie JG, et al. Densities and numbers of calbindin and parvalbumin positive neurons across the rat and mouse brain. IScience. 2021;24:101906.
Lupori L, Totaro V, Cornuti S, Ciampi L, Carrara F, Grilli E, et al. A comprehensive atlas of perineuronal net distribution and colocalization with parvalbumin in the adult mouse brain. Cell Rep. 2023;42:112788.
Härtig W, Derouiche A, Welt K, Brauer K, Grosche J, Mäder M, et al. Cortical neurons immunoreactive for the potassium channel Kv3.1b subunit are predominantly surrounded by perineuronal nets presumed as a buffering system for cations. Brain Res. 1999;842:15–29.
Carceller H, Guirado R, Ripolles-Campos E, Teruel-Marti V, Nacher J. Perineuronal nets regulate the inhibitory perisomatic input onto parvalbumin interneurons and γ activity in the prefrontal cortex. J Neurosci. 2020;40:5008–18.
Hayani H, Song I, Dityatev A. Increased excitability and reduced excitatory synaptic input into fast-spiking CA2 interneurons after enzymatic attenuation of extracellular matrix. Front Cell Neurosci. 2018;12:149.
Lensjø KK, Christensen AC, Tennøe S, Fyhn M, Hafting T. Differential expression and cell-type specificity of perineuronal nets in hippocampus, medial entorhinal cortex, and visual cortex examined in the rat and mouse. ENeuro. 2017;4:ENEURO.0379-16.2017.
Buyukata C, Vukalo M, Xu TJ, Khore MA, Reichelt AC. Impact of high sucrose diets on the discrimination of spatial and object memories with overlapping features. Physiol Behav. 2018;192:127–33.
Woodward EM, Coutellier L. Age- and sex-specific effects of stress on parvalbumin interneurons in preclinical models: Relevance to sex differences in clinical neuropsychiatric and neurodevelopmental disorders. Neurosci Biobehav Rev. 2021;131:1228–42.
Hernández-Vivanco A, de la Vega-Ruiz R, Montes-Mellado A, Azcoitia Í, Méndez P. Activational and organizational effects of sex hormones on hippocampal inhibitory neurons. J Neurosci. 2025;45:e1764242025.
Miller LR, Marks C, Becker JB, Hurn PD, Chen WJ, Woodruff T, et al. Considering sex as a biological variable in preclinical research. FASEB J. 2017;31:29–34.
Acknowledgements
We thank Prof Vania Prado, Prof Marco Prado and the Robarts Rodent Core for their assistance and acquisition of the transgenic mice, Mr Chris Fodor for his assistance with genotyping and colony breeding, and Prof Anthony Hannan for his feedback on the manuscript.
Funding
This research was supported by BrainsCAN at Western University through the Canada First Research Excellence Fund (CFREF) and the Natural Sciences and Engineering Research Council (NSERC). LMS is the Canada Research Chair in Translational Cognitive Neuroscience and TJB is the Western Research Chair in Behavioral Neuroscience. ACR received funding from the Australian Research Council (DP180101974).
Author information
Authors and Affiliations
Contributions
ACR, TJB, LMS, CPK, AMH and WI designed the experiments. ACR, AMH, MGS and OGS performed the experiments. ACR, OPL, GAS, and MM completed the immunohistochemistry and microscopy validation. ACR, OGS, TJB, LMS, AMH and WI wrote the manuscript. OGS edited and revised the manuscript and figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Reichelt, A.C., Hashad, A.M., Ghosh-Swaby, O.R. et al. A dual role of hippocampal perineuronal nets in plasticity and protection is revealed by improvement and impairment of diet-induced memory dysfunction. Neuropsychopharmacol. (2026). https://doi.org/10.1038/s41386-026-02343-z
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41386-026-02343-z