Abstract
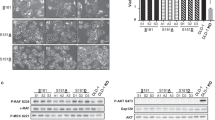
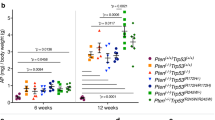
The serine protease PRSS8 has shown important physiological and pathological functions, but its roles in cancer initiation and progression are unclear. We developed and dynamically characterized a conditional knockout Prss8fl/fl, p-Villin-Cre+ mouse model. We found that genetic deficiency of the Prss8 gene caused spontaneous colitis and an inflamed rectum at an early age and caused intestinal tumors at a late age, which were linked to increased intestinal cell proliferation and migration but decreased cell differentiation. Increased PRSS8 expression inhibited cancer cell growth and metastasis in nude mice and inhibited cancer cell migration, invasion, colony formation and tumor sphere formation in vitro, but decreased PRSS8 expression facilitated malignancies in vivo and in vitro. Gene profiling on manipulated cancer cells and intestinal epithelial cells of Prss8 mouse models, gene set enrichment analysis and mechanistic studies revealed that PRSS8 targeted the Wnt/β-catenin, epithelial-mesenchymal transition, and stem cell signaling pathways, which were further supported by the results from the TCGA data mining and validated by immunohistochemical staining on colorectal cancer tissue microarrays. In conclusion, PRSS8 is a novel tumor suppressor that plays critical roles in the suppression of colorectal carcinogenesis and metastasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout












Similar content being viewed by others
Change history
22 February 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41388-021-01646-3
References
Yu JX, Chao L, Chao J. Prostasin is a novel human serine proteinase from seminal fluid. Purification, tissue distribution, and localization in prostate gland. J Biol Chem. 1994;269:18843–8.
Hooper JD, Bowen N, Marshall H, Cullen LM, Sood R, Daniels R, et al. Localization, expression and genomic structure of the gene encoding the human serine protease testisin. Biochim Biophys Acta. 2000;1492:63–71.
Yu JX, Chao L, Ward DC, Chao J. Structure and chromosomal localization of the human prostasin (PRSS8) gene. Genomics. 1996;32:334–40.
Uchimura K, Hayata M, Mizumoto T, Miyasato Y, Kakizoe Y, Morinaga J, et al. The serine protease prostasin regulates hepatic insulin sensitivity by modulating TLR4 signalling. Nat Commun. 2014;5:3428.
Lopez-Otin C, Matrisian LM. Emerging roles of proteases in tumour suppression. Nat Rev. 2007;7:800–8.
Sarojini S, Tamir A, Lim H, Li S, Zhang S, Goy A, et al. Early detection biomarkers for ovarian cancer. J Oncol. 2012;2012:709049.
Mok SC, Chao J, Skates S, Wong K, Yiu GK, Muto MG, et al. Prostasin, a potential serum marker for ovarian cancer: identification through microarray technology. J Natl Cancer Inst. 2001;93:1458–64.
Chen LM, Zhang X, Chai KX. Regulation of prostasin expression and function in the prostate. Prostate. 2004;59:1–12.
Takahashi S, Suzuki S, Inaguma S, Ikeda Y, Cho YM, Hayashi N, et al. Down-regulated expression of prostasin in high-grade or hormone-refractory human prostate cancers. Prostate. 2003;54:187–93.
Chen LM, Chai KX. Prostasin serine protease inhibits breast cancer invasiveness and is transcriptionally regulated by promoter DNA methylation. Int J Cancer. 2002;97:323–9.
Chen LM, Verity NJ, Chai KX. Loss of prostasin (PRSS8) in human bladder transitional cell carcinoma cell lines is associated with epithelial-mesenchymal transition (EMT). BMC Cancer. 2009;9:377.
Sakashita K, Mimori K, Tanaka F, Tahara K, Inoue H, Sawada T, et al. Clinical significance of low expression of Prostasin mRNA in human gastric cancer. J Surg Oncol. 2008;98:559–64.
Bao Y, Li K, Guo Y, Wang Q, Li Z, Yang Y, et al. Tumor suppressor PRSS8 targets Sphk1/S1P/Stat3/Akt signaling in colorectal cancer. Oncotarget. 2016;7:26780–92.
Bao Y, Wang Q, Guo Y, Chen Z, Li K, Yang Y, et al. PRSS8 methylation and its significance in esophageal squamous cell carcinoma. Oncotarget. 2016;7:28540–55.
Keppner A, Malsure S, Nobile A, Auberson M, Bonny O, Hummler E. Altered prostasin (CAP1/Prss8) expression favors inflammation and tissue remodeling in DSS-induced colitis. Inflamm Bowel Dis. 2016;22:2824–39.
Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, et al. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–9.
Bao Y, Guo Y, Li Z, Fang W, Yang Y, Li X, et al. MicroRNA profiling in Muc2 knockout mice of colitis-associated cancer model reveals epigenetic alterations during chronic colitis malignant transformation. PLoS ONE. 2014;9:e99132.
Wong NA, Pignatelli M. Β-catenin–a linchpin in colorectal carcinogenesis? Am J Pathol. 2002;160:389–401.
Aoki K, Taketo MM. Adenomatous polyposis coli (APC): a multi-functional tumor suppressor gene. J Cell Sci. 2007;120:3327–35.
Mokry M, Hatzis P, Schuijers J, Lansu N, Ruzius FP, Clevers H, et al. Integrated genome-wide analysis of transcription factor occupancy, RNA polymerase II binding and steady-state RNA levels identify differentially regulated functional gene classes. Nucleic Acids Res. 2012;40:148–58.
Paoni NF, Feldman MW, Gutierrez LS, Ploplis VA, Castellino FJ. Transcriptional profiling of the transition from normal intestinal epithelia to adenomas and carcinomas in the APCMin/+mouse. Physiol Genom. 2003;15:228–35.
Lee SY, Jeong EK, Ju MK, Jeon HM, Kim MY, Kim CH, et al. Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation. Mol Cancer. 2017;16:10.
TCGA. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–7.
Rodriguez-Salas N, Dominguez G, Barderas R, Mendiola M, Garcia-Albeniz X, Maurel J, et al. Clinical relevance of colorectal cancer molecular subtypes. Crit Rev Oncol Hematol. 2017;109:9–19.
Buchanan FG, DuBois RN. Connecting COX-2 and Wnt in cancer. Cancer Cell. 2006;9:6–8.
Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99.
Grivennikov SI. Inflammation and colorectal cancer: colitis-associated neoplasia. Semin Immunopathol. 2013;35:229–44.
Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21:11–19.
Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol. 2012;12:715–23.
Yang K, Popova NV, Yang WC, Lozonschi I, Tadesse S, Kent S, et al. Interaction of Muc2 and Apc on Wnt signaling and in intestinal tumorigenesis: potential role of chronic inflammation. Cancer Res. 2008;68:7313–22.
Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr., et al. Cancer genome landscapes. Science. 2013;339:1546–58.
Doki Y, Shiozaki H, Tahara H, Inoue M, Oka H, Iihara K, et al. Correlation between E-cadherin expression and invasiveness in vitro in a human esophageal cancer cell line. Cancer Res. 1993;53:3421–6.
Oka H, Shiozaki H, Kobayashi K, Inoue M, Tahara H, Kobayashi T, et al. Expression of E-cadherin cell adhesion molecules in human breast cancer tissues and its relationship to metastasis. Cancer Res. 1993;53:1696–701.
Umbas R, Isaacs WB, Bringuier PP, Schaafsma HE, Karthaus HF, Oosterhof GO, et al. Decreased E-cadherin expression is associated with poor prognosis in patients with prostate cancer. Cancer Res. 1994;54:3929–33.
Derksen PW, Liu X, Saridin F, van der Gulden H, Zevenhoven J, Evers B, et al. Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell. 2006;10:437–49.
Frixen UH, Behrens J, Sachs M, Eberle G, Voss B, Warda A, et al. E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol. 1991;113:173–85.
Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68:3645–54.
Baranwal S, Alahari SK. Molecular mechanisms controlling E-cadherin expression in breast cancer. Biochem Biophys Res Commun. 2009;384:6–11.
Schmalhofer O, Brabletz S, Brabletz T. E-cadherin, β-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastas Rev. 2009;28:151–66.
Peters DE, Szabo R, Friis S, Shylo NA, Uzzun Sales K, Holmbeck K, et al. The membrane-anchored serine protease prostasin (CAP1/PRSS8) supports epidermal development and postnatal homeostasis independent of its enzymatic activity. J Biol Chem. 2014;289:14740–9.
Beck B, Blanpain C. Unravelling cancer stem cell potential. Nat Rev. 2013;13:727–38.
Ordonez-Moran P, Dafflon C, Imajo M, Nishida E, Huelsken J. HOXA5 counteracts stem cell traits by inhibiting Wnt signaling in colorectal cancer. Cancer Cell. 2015;28:815–29.
Sansom OJ, Meniel VS, Muncan V, Phesse TJ, Wilkins JA, Reed KR, et al. Myc deletion rescues Apc deficiency in the small intestine. Nature. 2007;446:676–9.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–50.
Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30.
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29.
Hu D, Fang W, Han A, Gallagher L, Davis RJ, Xiong B, et al. c-Jun N-terminal kinase 1 interacts with and negatively regulates Wnt/β-catenin signaling through GSK3β pathway. Carcinogenesis. 2008;29:2317–24.
Bi X, Pohl NM, Qian Z, Yang GR, Gou Y, Guzman G, et al. Decorin-mediated inhibition of colorectal cancer growth and migration is associated with E-cadherin in vitro and in mice. Carcinogenesis. 2012;33:326–30.
Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–9.
Liu JC, Deng T, Lehal RS, Kim J, Zacksenhaus E. Identification of tumorsphere- and tumor-initiating cells in HER2/Neu-induced mammary tumors. Cancer Res. 2007;67:8671–81.
Acknowledgements
This work was supported in part by grants from the National Nature Science Foundation of China (grants 81672750 and 91229115 to WY, grant 81502105 to YB), a grant from the Key Laboratory of Higher Education Institutes of Shandong Province, the Nature Science Foundation of Shandong Province (grant 2016ZRB14436 to YG) and Taishan Scholar Program of Shandong Province, China, a grant from the Innovation Team of Science and Technology, Henan Province, China, the Startup Fund from Jining Medical University (to YB and YG) and Fostering fund from Jining Medical University (to YG). We would like to thank Prof. Weixing Zhao (Xinxiang Medical University, Xinxiang, China), Prof. Renya Zhang (Jining Medical University, Jining, China) and Prof. Anjia Han (Sun Yat-sen University, Guangzhou, China) for assistance with the histopathology analysis of the mouse models, Prof. Alan Diamond (University of Illinois at Chicago, Chicago, USA) for critical discussion, and Springer Nature Author Services for editing this manuscript. We would also like to thank the Research Histology and Tissue Imaging Core (RHTIC) facility at the University of Illinois at Chicago (Chicago, IL) for their technical assistance.
Author contributions:
Study concept and design: WY, YB; acquisition of data: YB, YG, YY, XW, SZ, YZ, KL, MY, SC; analysis and interpretation of the data: WY, YB, YG; technical and material support: DG, XZ; statistical analysis: YG, WZ; study supervision: WY, YB; drafting of the manuscript: WY; funding: WY, YB, YG, WZ. All authors contributed to critical revision of the manuscript and approved the final version.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Bao, Y., Guo, Y., Yang, Y. et al. PRSS8 suppresses colorectal carcinogenesis and metastasis. Oncogene 38, 497–517 (2019). https://doi.org/10.1038/s41388-018-0453-3
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41388-018-0453-3
This article is cited by
-
Exploring the link between M1 macrophages and EMT of amniotic epithelial cells: implications for premature rupture of membranes
Journal of Nanobiotechnology (2025)
-
Unveiling the protein landscape for early detection of colorectal precancerous lesions
Clinical Proteomics (2025)
-
The cell-surface serine protease prostasin is lost during cervical squamous cell carcinogenesis
Human Cell (2025)
-
Serine protease PRSS56, a novel cancer-testis antigen activated by DNA hypomethylation, promotes colorectal and gastric cancer progression via PI3K/AKT axis
Cell & Bioscience (2023)
-
The potential oncogenic effect of tissue-specific expression of JC polyoma T antigen in digestive epithelial cells
Transgenic Research (2023)