Abstract
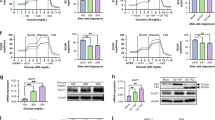
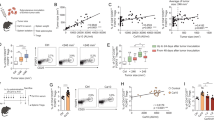
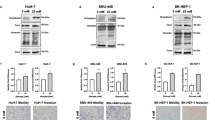
Diabetes mellitus (DM) characterized by hyperglycemia is a chronic metabolic disorder that leads to many symptoms and vascular complications. Despite the close association between DM and cancer progression, the response and role of endothelial cells (ECs) under diabetic conditions in tumor metastasis remain to be elucidated. In this study, we sought to determine whether and how ECs under diabetic conditions contribute to tumor metastasis. We have taken advantage of syngeneic mouse tumor models of Lewis lung carcinoma (LLC) and melanoma (B16F10) cells and a streptozotocin (STZ)-induced hyperglycemia model. We demonstrated that hyperglycemia increased the metastasis of LLC and B16F10 cells in an experimental metastasis model with an intravenous injection of the tumor cells. We also found that hyperglycemia promoted lung metastasis of tumor cells by increasing the adhesiveness of ECs to facilitate the adhesion of tumor cells to ECs rather than affecting the metastatic behavior of tumor cells themselves. From the analysis of gene expression in primary lung ECs from STZ-treated mice, we identified that vWF promoted the adhesion of tumor cells to ECs and the transendothelial migration of tumor cells. Mechanistically, hyperglycemia-induced oxidative stress in ECs, and increased oxidative stress enhanced vWF expression in ECs through an increase in the transcription factor GATA1. These results provide evidence for the role of vWF in ECs in promoting hyperglycemia-induced tumor metastasis and potential therapeutic targets for the regulation of vWF expression in ECs and hyperglycemia-induced tumor metastasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–18.
Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904.
Orr FW, Wang HH, Lafrenie RM, Scherbarth S, Nance DM. Interactions between cancer cells and the endothelium in metastasis. J Pathol. 2000;190:310–29.
Carstensen B, Read SH, Friis S, Sund R, Keskimaki I, Svensson AM, et al. Cancer incidence in persons with type 1 diabetes: a five-country study of 9,000 cancers in type 1 diabetic individuals. Diabetologia. 2016;59:980–8.
Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, et al. Diabetes and cancer: a consensus report. CA: Cancer J Clin. 2010;60:207–21.
Noh Y, Jeon SM, Shin S. Association between glucose-lowering treatment and cancer metastasis among patients with preexisting type 2 diabetes and incident malignancy. Int J Cancer. 2019;144:1530–9.
Spratt DE, Beadle BM, Zumsteg ZS, Rivera A, Skinner HD, Osborne JR, et al. The influence of diabetes mellitus and metformin on distant metastases in oropharyngeal cancer: a multicenter study. Int J Radiat Oncol Biol Phys. 2016;94:523–31.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33.
Scappaticcio L, Maiorino MI, Bellastella G, Giugliano D, Esposito K. Insights into the relationships between diabetes, prediabetes, and cancer. Endocrine. 2017;56:231–9.
Fainsod-Levi T, Gershkovitz M, Vols S, Kumar S, Khawaled S, Sagiv JY, et al. Hyperglycemia impairs neutrophil mobilization leading to enhanced metastatic seeding. Cell Rep. 2017;21:2384–92.
Jafar N, Edriss H, Nugent K. The effect of short-term hyperglycemia on the innate immune system. Am J Med Sci. 2016;351:201–11.
Ikemura M, Nishikawa M, Kusamori K, Fukuoka M, Yamashita F, Hashida M. Pivotal role of oxidative stress in tumor metastasis under diabetic conditions in mice. J Controlled Release. 2013;170:191–7.
Basta G, Schmidt AM, De, Caterina R. Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc Res. 2004;63:582–92.
Franses JW, Drosu NC, Gibson WJ, Chitalia VC, Edelman ER. Dysfunctional endothelial cells directly stimulate cancer inflammation and metastasis. Int J Cancer. 2013;133:1334–44.
Jeon HY, Lee YJ, Kim YS, Kim SY, Han ET, Park WS, et al. Proinsulin C-peptide prevents hyperglycemia-induced vascular leakage and metastasis of melanoma cells in the lungs of diabetic mice. FASEB J. 2019;33:750–62.
Sadler JE. von Willebrand factor in its native environment. Blood. 2013;121:2583–4.
Nichols TC, Samama CM, Bellinger DA, Roussi J, Reddick RL, Bonneau M, et al. Function of von Willebrand factor after crossed bone marrow transplantation between normal and von Willebrand disease pigs: effect on arterial thrombosis in chimeras. Proc Natl Acad Sci USA. 1995;92:2455–9.
Wagner DD. Cell biology of von Willebrand factor. Annu Rev Cell Biol. 1990;6:217–46.
De Ceunynck K, De Meyer SF, Vanhoorelbeke K. Unwinding the von Willebrand factor strings puzzle. Blood. 2013;121:270–7.
Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11:123–34.
Kim KJ, Kwon SH, Yun JH, Jeong HS, Kim HR, Lee EH, et al. STAT3 activation in endothelial cells is important for tumor metastasis via increased cell adhesion molecule expression. Oncogene. 2017;36:5445–59.
Yun JH, Lee DH, Jeong HS, Kim HS, Ye SK, Cho CH. STAT3 activation in microglia exacerbates hippocampal neuronal apoptosis in diabetic brains. J Cell Physiol. 2021;236:7058–70.
Wang J, Niu N, Xu S, Jin ZG. A simple protocol for isolating mouse lung endothelial cells. Sci Rep. 2019;9:1458.
Yun JH, Park SW, Kim KJ, Bae JS, Lee EH, Paek SH, et al. Endothelial STAT3 activation increases vascular leakage through downregulating tight junction proteins: implications for diabetic retinopathy. J Cell Physiol. 2017;232:1123–34.
Terraube V, Marx I, Denis CV. Role of von Willebrand factor in tumor metastasis. Thromb Res. 2007;120:S64–70.
Marini A, Rotblat B, Sbarrato T, Niklison-Chirou MV, Knight JRP, Dudek K, et al. TAp73 contributes to the oxidative stress response by regulating protein synthesis. Proc Natl Acad Sci USA. 2018;115:6219–24.
Chan SH, Wu CA, Wu KL, Ho YH, Chang AY, Chan JY. Transcriptional upregulation of mitochondrial uncoupling protein 2 protects against oxidative stress-associated neurogenic hypertension. Circ Res. 2009;105:886–96.
Xiang Y, Cheng J, Wang D, Hu X, Xie Y, Stitham J, et al. Hyperglycemia repression of miR-24 coordinately upregulates endothelial cell expression and secretion of von Willebrand factor. Blood. 2015;125:3377–87.
Lou Z, Li X, Zhao X, Du K, Wang B. Resveratrol attenuates hydrogen peroxideinduced apoptosis, reactive oxygen species generation, and PSGL1 and VWF activation in human umbilical vein endothelial cells, potentially via MAPK signalling pathways. Mol Med Rep. 2018;17:2479–87.
Li JM, Mullen AM, Yun S, Wientjes F, Brouns GY, Thrasher AJ, et al. Essential role of the NADPH oxidase subunit p47(phox) in endothelial cell superoxide production in response to phorbol ester and tumor necrosis factor-alpha. Circ Res. 2002;90:143–50.
He H, Wang L, Qiao Y, Zhou Q, Li H, Chen S, et al. Doxorubicin induces endotheliotoxicity and mitochondrial dysfunction via ROS/eNOS/NO pathway. Front Pharm. 2019;10:1531.
Liu J, Kanki Y, Okada Y, Jin E, Yano K, Shih SC, et al. A +220 GATA motif mediates basal but not endotoxin-repressible expression of the von Willebrand factor promoter in Hprt-targeted transgenic mice. J Thromb Haemost. 2009;7:1384–92.
Schwachtgen JL, Janel N, Barek L, Duterque-Coquillaud M, Ghysdael J, Meyer D, et al. Ets transcription factors bind and transactivate the core promoter of the von Willebrand factor gene. Oncogene. 1997;15:3091–102.
Dmitrieva NI, Burg MB. Secretion of von Willebrand factor by endothelial cells links sodium to hypercoagulability and thrombosis. Proc Natl Acad Sci USA. 2014;111:6485–90.
Li WJ, Zhang XH, Sang H, Zhou Y, Shang CY, Wang YQ, et al. Effects of hyperglycemiaon the progression of tumor diseases. J Exp Clin Canc Res. 2019;38:327–33.
Ikemura M, Hashida T. Effect of hyperglycemia on antitumor activity and survival in tumor-bearing mice receiving oxaliplatin and fluorouracil. Anticancer Res. 2017;37:5463–8.
Foda HD, Zucker S. Matrix metalloproteinases in cancer invasion, metastasis and angiogenesis. Drug Discov Today. 2001;6:478–82.
Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709.
Zheng Y, Yamamoto S, Ishii Y, Sang Y, Hamashima T, Van De N, et al. Glioma-derived platelet-derived growth factor-bb recruits oligodendrocyte progenitor cells via platelet-derived growth factor receptor-alpha and remodels cancer stroma. Am J Pathol. 2016;186:1081–91.
Aghajanian A, Wittchen ES, Allingham MJ, Garrett TA, Burridge K. Endothelial cell junctions and the regulation of vascular permeability and leukocyte transmigration. J Thromb Haemost. 2008;6:1453–60.
Garcia-Roman J, Zentella-Dehesa A. Vascular permeability changes involved in tumor metastasis. Cancer Lett. 2013;335:259–69.
Drummond GR, Sobey CG. Endothelial NADPH oxidases: which NOX to target in vascular disease? Trends Endocrinol Metab. 2014;25:452–63.
Gorin Y, Block K. Nox as a target for diabetic complications. Clin Sci. 2013;125:361–82.
Dymkowska D, Drabarek B, Podszywalow-Bartnicka P, Szczepanowska J, Zablocki K. Hyperglycaemia modifies energy metabolism and reactive oxygen species formation in endothelial cells in vitro. Arch Biochem Biophys. 2014;542:7–13.
Avdonin PV, Rybakova EY, Avdonin PP, Trufanov SK, Mironova GY, Tsitrina AA, et al. VAS2870 inhibits histamine-induced calcium signaling and vWF secretion in human umbilical vein endothelial cells. Cells. 2019;8:196–207.
Vischer UM, Jornot L, Wollheim CB, Theler JM. Reactive oxygen intermediates induce regulated secretion of von Willebrand factor from cultured human vascular endothelial cells. Blood. 1995;85:3164–72.
Takano M, Meneshian A, Sheikh E, Yamakawa Y, Wilkins KB, Hopkins EA, et al. Rapid upregulation of endothelial P-selectin expression via reactive oxygen species generation. Am J Physiol Heart Circ Physiol. 2002;283:H2054–2061.
Mordes DB, Lazarchick J, Colwell JA, Sens DA. Elevated glucose concentrations increase factor VIIIR:Ag levels in human umbilical vein endothelial cells. Diabetes. 1983;32:876–8.
Chan NN, Fuller JH, Rubens M, Colhoun HM. Von Willebrand factor in type 1 diabetes: its production and coronary artery calcification. Med Sci Monit: Int Med J Exp Clin Res. 2003;9:CR297–303.
Seligman BG, Biolo A, Polanczyk CA, Gross JL, Clausell N. Increased plasma levels of endothelin 1 and von Willebrand factor in patients with type 2 diabetes and dyslipidemia. Diabetes Care. 2000;23:1395–1400.
Umetani M, Mataki C, Minegishi N, Yamamoto M, Hamakubo T, Kodama T. Function of GATA transcription factors in induction of endothelial vascular cell adhesion molecule-1 by tumor necrosis factor-alpha. Arteriosc Thromb Vasc Biol. 2001;21:917–22.
Fan C, Ouyang P, Timur AA, He P, You SA, Hu Y, et al. Novel roles of GATA1 in regulation of angiogenic factor AGGF1 and endothelial cell function. J Biol Chem. 2009;284:23331–43.
O’Sullivan JM, Preston RJS, Robson T, O’Donnell JS. Emerging roles for von Willebrand factor in cancer cell biology. Semin Thromb Hemost. 2018;44:159–66.
Gadducci A, Baicchi U, Marrai R, Del Bravo B, Fosella PV, Facchini V. Pretreatment plasma levels of fibrinopeptide-A (FPA), D-dimer (DD), and von Willebrand factor (vWF) in patients with ovarian carcinoma. Gynecologic Oncol. 1994;53:352–6.
Blann AD, Balakrishnan B, Shantsila E, Ryan P, Lip GY. Endothelial progenitor cells and circulating endothelial cells in early prostate cancer: a comparison with plasma vascular markers. Prostate. 2011;71:1047–53.
Rohsig LM, Damin DC, Stefani SD, Castro CG Jr, Roisenberg I, Schwartsmann G. von Willebrand factor antigen levels in plasma of patients with malignant breast disease. Braz J Med Biol Res. 2001;34:1125–9.
Wang WS, Lin JK, Lin TC, Chiou TJ, Liu JH, Yen CC, et al. Plasma von Willebrand factor level as a prognostic indicator of patients with metastatic colorectal carcinoma. World J Gastroenterol. 2005;11:2166–70.
Franchini M, Frattini F, Crestani S, Bonfanti C, Lippi G. von Willebrand factor and cancer: a renewed interest. Thromb Res. 2013;131:290–2.
Li N. Platelets in cancer metastasis: to help the “villain” to do evil. Int J Cancer. 2016;138:2078–87.
Pilch J, Habermann R, Felding-Habermann B. Unique ability of integrin alpha(v)beta 3 to support tumor cell arrest under dynamic flow conditions. J Biol Chem. 2002;277:21930–8.
Coller BS, Shattil SJ. The GPIIb/IIIa (integrin alphaIIbbeta3) odyssey: a technology-driven saga of a receptor with twists, turns, and even a bend. Blood. 2008;112:3011–25.
Terraube V, Pendu R, Baruch D, Gebbink MF, Meyer D, Lenting PJ, et al. Increased metastatic potential of tumor cells in von Willebrand factor-deficient mice. J Thromb Haemost: JTH. 2006;4:519–26.
Karpatkin S, Pearlstein E, Ambrogio C, Coller BS. Role of adhesive proteins in platelet tumor interaction in vitro and metastasis formation in vivo. J Clin Investig. 1988;81:1012–9.
Morganti M, Carpi A, Amo-Takyi B, Sagripanti A, Nicolini A, Giardino R, et al. Von Willebrand’s factor mediates the adherence of human tumoral cells to human endothelial cells and ticlopidine interferes with this effect. Biomed Pharmacother. 2000;54:431–6.
Acknowledgements
We appreciate Dr. Kyong Soo Park for technical assistance in insulin supplementation experiments. This work is supported by funding from the National Research Foundation of Korea grant funded by the Korea government (2020R1A2C1008008 to CHC) and the SNUH Research Fund (0420210290 to CHC).
Author information
Authors and Affiliations
Contributions
HSJ performed majority of the experiments, analyzed the results, and wrote the manuscript. CHC (corresponding author) supervised the project and wrote the manuscript. DHL and SHK assisted with all experiments and reviewed the manuscript. HMS and HRK performed RNA sequencing, pathway analysis, and reviewed the manuscript. CHL helped in project design, data analysis, and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Jeong, HS., Lee, DH., Kim, SH. et al. Hyperglycemia-induced oxidative stress promotes tumor metastasis by upregulating vWF expression in endothelial cells through the transcription factor GATA1. Oncogene 41, 1634–1646 (2022). https://doi.org/10.1038/s41388-022-02207-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41388-022-02207-y
This article is cited by
-
GATA1 controls metadherin transcription to promote oxidative stress-induced podocyte injury
Journal of Molecular Medicine (2026)
-
Influence of diabetes mellitus and anti-diabetic drugs on pathophysiology and prognosis for primary prostate cancer
International Urology and Nephrology (2026)
-
Immunometabolism: crosstalk with tumor metabolism and implications for cancer immunotherapy
Molecular Cancer (2025)
-
Comparative risk of cancer associated with SGLT inhibitors and DPP-4 inhibitors in patients with diabetes: a systematic review and meta-analysis
Diabetology & Metabolic Syndrome (2025)
-
Overexpression of MEOX2 inhibits breast cancer cell metastasis by targeting oxidative stress-induced RGS5
In Vitro Cellular & Developmental Biology - Animal (2025)