Abstract
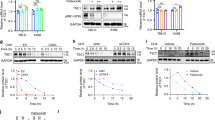
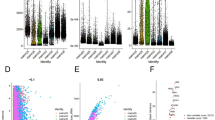
Clear cell renal cell carcinoma (ccRCC), the most common subtype of renal cell carcinoma, often exhibits resistance to tyrosine kinase inhibitors (TKIs) when used as monotherapy. However, the integration of PD-1 blockade with TKIs has significantly improved patient survival, making it a leading therapeutic strategy for ccRCC. Despite these advancements, the efficacy of this combined therapy remains suboptimal, necessitating a deeper understanding of the underlying regulatory mechanisms. Through comprehensive analyses, including mass spectrometry, RNA sequencing, lipidomic profiling, immunohistochemical staining, and ex vivo experiments, we explored the interaction between PTPRZ1 and RNF26 and its impact on ccRCC cell behavior. Our results revealed a unique interaction where PTPRZ1 stabilized RNF26 protein expression by dephosphorylating it at the Y432 site. The modulation of RNF26 levels by PTPRZ1 was found to be mediated through the proteasome pathway. Additionally, PTPRZ1, via its interaction with RNF26, activated the TNF/NF-κB signaling pathway, thereby promoting cell proliferation, angiogenesis, and lipid metabolism in ccRCC cells. Importantly, inhibiting PTPRZ1 enhanced the sensitivity of ccRCC to TKIs and PD-1 blockade, an effect that was attenuated when RNF26 was simultaneously knocked down. These findings highlight the critical role of the PTPRZ1-RNF26 axis in ccRCC and suggest that combining PTPRZ1 inhibitors with current TKIs and PD-1 blockade therapies could significantly improve treatment outcomes for ccRCC patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable request.
References
Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol. 2016;70:93–105.
Ljungberg B, Albiges L, Abu-Ghanem Y, Bensalah K, Dabestani S, Fernandez-Pello S, et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2019 Update. Eur Urol. 2019;75:799–810.
Karam JA, Devine CE, Urbauer DL, Lozano M, Maity T, Ahrar K, et al. Phase 2 trial of neoadjuvant axitinib in patients with locally advanced nonmetastatic clear cell renal cell carcinoma. Eur Urol. 2014;66:874–80.
Gossage L, Eisen T. Alterations in VHL as potential biomarkers in renal-cell carcinoma. Nat Rev Clin Oncol. 2010;7:277–88.
Motzer RJ, Porta C, Eto M, Powles T, Grunwald V, Hutson TE, et al. Lenvatinib Plus Pembrolizumab Versus Sunitinib in First-Line Treatment of Advanced Renal Cell Carcinoma: Final Prespecified Overall Survival Analysis of CLEAR, a Phase III Study. J Clin Oncol. 2024;42:1222–8.
Catalano M, De Giorgi U, Bimbatti D, Buti S, Procopio G, Sepe P, et al. Impact of Metastatic Site in Favorable-Risk Renal Cell Carcinoma Receiving Sunitinib or Pazopanib. Clin Genitourin Cancer. 2024;22:514–22.e511.
Kase AM, George DJ, Ramalingam S. Clear Cell Renal Cell Carcinoma: From Biology to Treatment. Cancers (Basel). 2023;15:665.
Vano YA, Elaidi R, Bennamoun M, Chevreau C, Borchiellini D, Pannier D, et al. Nivolumab, nivolumab-ipilimumab, and VEGFR-tyrosine kinase inhibitors as first-line treatment for metastatic clear-cell renal cell carcinoma (BIONIKK): a biomarker-driven, open-label, non-comparative, randomised, phase 2 trial. Lancet Oncol. 2022;23:612–24.
Cremer T, Jongsma MLM, Trulsson F, Vertegaal ACO, Neefjes J, Berlin I. The ER-embedded UBE2J1/RNF26 ubiquitylation complex exerts spatiotemporal control over the endolysosomal pathway. Cell Rep. 2021;34:108659.
Cremer T, Voortman LM, Bos E, Jongsma ML, Ter Haar LR, Akkermans JJ, et al. RNF26 binds perinuclear vimentin filaments to integrate ER and endolysosomal responses to proteotoxic stress. EMBO J. 2023;42:e111252.
Lu X, Zhang Y, Wu Y, Lu T, Yang H, Yang W, et al. RNF26 Promotes Pancreatic Cancer Proliferation by Enhancing RBM38 Degradation. Pancreas. 2022;51:1427–33.
Yi L, Wang H, Li W, Ye K, Xiong W, Yu H, et al. The FOXM1/RNF26/p57 axis regulates the cell cycle to promote the aggressiveness of bladder cancer. Cell Death Dis. 2021;12:944.
Liu W, Wang H, Jian C, Li W, Ye K, Ren J, et al. The RNF26/CBX7 axis modulates the TNF pathway to promote cell proliferation and regulate sensitivity to TKIs in ccRCC. Int J Biol Sci. 2022;18:2132–45.
He ZC, Liu Q, Yang KD, Chen C, Zhang XN, Wang WY, et al. HOXA5 is amplified in glioblastoma stem cells and promotes tumor progression by transcriptionally activating PTPRZ1. Cancer Lett. 2022;533:215605.
Lorente G, Nelson A, Mueller S, Kuo J, Urfer R, Nikolich K, et al. Functional comparison of long and short splice forms of RPTPbeta: implications for glioblastoma treatment. Neuro Oncol. 2005;7:154–63.
Nagai K, Fujii M, Kitazume S. Protein Tyrosine Phosphatase Receptor Type Z in Central Nervous System Disease. Int J Mol Sci. 2022;23:4414.
Shang D, Xu X, Wang D, Li Y, Liu Y. Protein tyrosine phosphatase zeta enhances proliferation by increasing beta-catenin nuclear expression in VHL-inactive human renal cell carcinoma cells. World J Urol. 2013;31:1547–54.
Wang V, Davis DA, Haque M, Huang LE, Yarchoan R. Differential gene up-regulation by hypoxia-inducible factor-1alpha and hypoxia-inducible factor-2alpha in HEK293T cells. Cancer Res. 2005;65:3299–306.
Liu YT, Shang D, Akatsuka S, Ohara H, Dutta KK, Mizushima K, et al. Chronic oxidative stress causes amplification and overexpression of ptprz1 protein tyrosine phosphatase to activate beta-catenin pathway. Am J Pathol. 2007;171:1978–88.
Fenech EJ, Lari F, Charles PD, Fischer R, Laetitia-Thezenas M, Bagola K, et al. Interaction mapping of endoplasmic reticulum ubiquitin ligases identifies modulators of innate immune signalling. Elife. 2020;9:e57306.
Ko PJ, Dixon SJ. Protein palmitoylation and cancer. EMBO Rep. 2018;19:e46666.
Peng Z, Cheng S, Kou Y, Wang Z, Jin R, Hu H, et al. The Gut Microbiome Is Associated with Clinical Response to Anti-PD-1/PD-L1 Immunotherapy in Gastrointestinal Cancer. Cancer Immunol Res. 2020;8:1251–61.
Li Y, Zhang L, Ren P, Yang Y, Li S, Qin X, et al. Qing-Xue-Xiao-Zhi formula attenuates atherosclerosis by inhibiting macrophage lipid accumulation and inflammatory response via TLR4/MyD88/NF-κB pathway regulation. Phytomedicine. 2021;93:153812.
Xu P, Sun Z, Wang Y, Miao C. Long-term use of indomethacin leads to poor prognoses through promoting the expression of PD-1 and PD-L2 via TRIF/NF-κB pathway and JAK/STAT3 pathway to inhibit TNF-α and IFN-γ in hepatocellular carcinoma. Exp Cell Res. 2015;337:53–60.
Goldmann T, Otto F, Vollmer E. A receptor-type protein tyrosine phosphatase PTP zeta is expressed in human cutaneous melanomas. Folia Histochem Cytobiol. 2000;38:19–20.
Fu F, Xiao XI, Zhang T, Zou Q, Chen Z, Pei L, et al. Expression of receptor protein tyrosine phosphatase zeta is a risk factor for triple negative breast cancer relapse. Biomed Rep. 2016;4:167–72.
Ma Y, Ye F, Xie X, Zhou C, Lu W. Significance of PTPRZ1 and CIN85 expression in cervical carcinoma. Arch Gynecol Obstet. 2011;284:699–704.
Sethi G, Kwon Y, Burkhalter RJ, Pathak HB, Madan R, McHugh S, et al. PTN signaling: Components and mechanistic insights in human ovarian cancer. Mol Carcinog. 2015;54:1772–85.
Yamakawa T, Kurosawa N, Kadomatsu K, Matsui T, Itoh K, Maeda N, et al. Levels of expression of pleiotrophin and protein tyrosine phosphatase zeta are decreased in human colorectal cancers. Cancer Lett. 1999;135:91–6.
Laczmanska I, Karpinski P, Bebenek M, Sedziak T, Ramsey D, Szmida E, et al. Protein tyrosine phosphatase receptor-like genes are frequently hypermethylated in sporadic colorectal cancer. J Hum Genet. 2013;58:11–5.
Xia Z, Ouyang D, Li Q, Li M, Zou Q, Li L, et al. The Expression, Functions, Interactions and Prognostic Values of PTPRZ1: A Review and Bioinformatic Analysis. J Cancer. 2019;10:1663–74.
Ma L, Shen T, Peng H, Wu J, Wang W, Gao X. Overexpression of PTPRZ1 Regulates p120/beta-Catenin Phosphorylation to Promote Carcinogenesis of Oral Submucous Fibrosis. J Oncol. 2022;2022:2352360.
Kastana P, Ntenekou D, Mourkogianni E, Enake MK, Xanthopoulos A, Choleva E, et al. Genetic deletion or tyrosine phosphatase inhibition of PTPRZ1 activates c-Met to up-regulate angiogenesis and lung adenocarcinoma growth. Int J Cancer. 2023;153:1051–66.
Acknowledgements
We would like to thank ProMab Biotechnologies, Inc. (Changsha, China) for their support with western blotting. Our schematic diagrams were created with BioRender.com.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 82203537 (Wentao Liu)).
Author information
Authors and Affiliations
Contributions
Yongkang Ma: Methodology; Wei Li: Methodology; Xinlin Liu: Methodology; Weilin Peng: Formal analysis; Bei Qing: Formal analysis; Shangqing Ren: Project administration; Wentao Liu: Methodology, Investigation; Xiaobing Chen: Project administration, Investigation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was conducted in accordance with the principles of the Declaration of Helsinki principles. It was approved by the Animal Use and Care Committees at the Second Xiangya hospital, Central South University.
Consent to publication
All subjects have written informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, Y., Li, W., Liu, X. et al. PTPRZ1 dephosphorylates and stabilizes RNF26 to reduce the efficacy of TKIs and PD-1 blockade in ccRCC. Oncogene 43, 3633–3644 (2024). https://doi.org/10.1038/s41388-024-03198-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41388-024-03198-8