Abstract
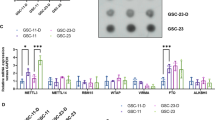
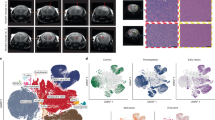
Inducing tumor cell differentiation is a promising strategy for treating malignant cancers, including glioma, yet the critical regulator(s) underlying glioma cell differentiation is poorly understood. Here, we identify G Protein Subunit Alpha O1 (GNAO1) as a critical regulator of neural differentiation of glioma stem-like cells (GSCs). GNAO1 expression was lower in gliomas than in normal neuronal tissues and high expression of GNAO1 correlated with a better prognosis. GNAO1 overexpression markedly promoted neural differentiation of GSCs, leading to decreased cell proliferation and colony formation. Mechanistically, GNAO1 recruited TRIM21 and facilitated TRIM21-mediated ubiquitination. This ubiquitination resulted in the degradation of CREB and further reduced p300-mediated H3K27ac levels of the HES1 promoter. As a result, GNAO1 overexpression downregulated HES1 expression, which reinforced neuronal differentiation. In addition, knockdown of METTL3, a key writer of the N6-methyladenosine (m6A), enhanced GNAO1 mRNA stability. Treatment with GNAO1 adenovirus increased neuronal differentiation of tumor cells and reduced tumor cell proliferation in orthotopic GSC xenografts and temozolomide further enhanced GNAO1 adenovirus effects, resulting in extended animal survival. Our study presents that engineering GNAO1 overexpression-inducing neural differentiation of GSCs is a potential therapy strategy via synergistic inhibition of malignant proliferation and chemotherapy resistance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
RNA-Seq data reported in this study have been deposited with the Gene Expression Omnibus under the accession GEO ID: GSE234341. (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE234341). The data supporting the findings of this study are available within the article and its Supporting Information files or available from the corresponding author upon reasonable request.
References
Hausmann D, Hoffmann DC, Venkataramani V, Jung E, Horschitz S, Tetzlaff SK, et al. Autonomous rhythmic activity in glioma networks drives brain tumour growth. Nature. 2023;613:179–86.
Caccese M, Indraccolo S, Zagonel V, Lombardi G. PD-1/PD-L1 immune-checkpoint inhibitors in glioblastoma: A concise review. Crit Rev Oncol Hematol. 2019;135:128–34.
Maghrouni A, Givari M, Jalili-Nik M, Mollazadeh H, Bibak B, Sadeghi MM, et al. Targeting the PD-1/PD-L1 pathway in glioblastoma multiforme: Preclinical evidence and clinical interventions. Int Immunopharmacol. 2021;93:107403.
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109.
Chen YC, Ma NX, Pei ZF, Wu Z, Do-Monte FH, Keefe S, et al. A NeuroD1 AAV-Based Gene Therapy for Functional Brain Repair after Ischemic Injury through In Vivo Astrocyte-to-Neuron Conversion. Mol Ther. 2020;28:217–34.
Guo Z, Zhang L, Wu Z, Chen Y, Wang F, Chen G. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell. 2014;14:188–202.
Heinrich C, Bergami M, Gascon S, Lepier A, Vigano F, Dimou L, et al. Sox2-mediated conversion of NG2 glia into induced neurons in the injured adult cerebral cortex. Stem Cell Rep. 2014;3:1000–14.
Rohle D, Popovici-Muller J, Palaskas N, Turcan S, Grommes C, Campos C, et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science. 2013;340:626–30.
Wang X, Pei Z, Hossain A, Bai Y, Chen G. Transcription factor-based gene therapy to treat glioblastoma through direct neuronal conversion. Cancer Biol Med. 2021;18:860–74.
Xu J, Fang S, Deng S, Li H, Lin X, Huang Y, et al. Generation of neural organoids for spinal-cord regeneration via the direct reprogramming of human astrocytes. Nat Biomed Eng. 2022;7:253–69.
Nakamura K, Kodera H, Akita T, Shiina M, Kato M, Hoshino H, et al. De Novo mutations in GNAO1, encoding a Gαo subunit of heterotrimeric G proteins, cause epileptic encephalopathy. Am J Hum Genet. 2013;93:496–505.
Larasati YA, Savitsky M, Koval A, Solis GP, Valnohova J, Katanaev VL. Restoration of the GTPase activity and cellular interactions of Galpha(o) mutants by Zn(2+) in GNAO1 encephalopathy models. Sci Adv. 2022;8:eabn9350.
Strittmatter SM, Valenzuela D, Kennedy TE, Neer EJ, Fishman MC. G0 is a major growth cone protein subject to regulation by GAP-43. Nature. 1990;344:836–41.
Akamine S, Okuzono S, Yamamoto H, Setoyama D, Sagata N, Ohgidani M, et al. GNAO1 organizes the cytoskeletal remodeling and firing of developing neurons. Faseb j. 2020;34:16601–21.
Ghil SH, Kim BJ, Lee YD, Suh-Kim H. Neurite outgrowth induced by cyclic AMP can be modulated by the alpha subunit of Go. J Neurochem. 2000;74:151–8.
Saitsu H, Fukai R, Ben-Zeev B, Sakai Y, Mimaki M, Okamoto N, et al. Phenotypic spectrum of GNAO1 variants: epileptic encephalopathy to involuntary movements with severe developmental delay. Eur J Hum Genet. 2016;24:129–34.
Danti FR, Galosi S, Romani M, Montomoli M, Carss KJ, Raymond FL, et al. GNAO1 encephalopathy: Broadening the phenotype and evaluating treatment and outcome. Neurol Genet. 2017;3:e143.
Schorling DC, Dietel T, Evers C, Hinderhofer K, Korinthenberg R, Ezzo D, et al. Expanding Phenotype of De Novo Mutations in GNAO1: Four New Cases and Review of Literature. Neuropediatrics. 2017;48:371–7.
Gerald B, Ramsey K, Belnap N, Szelinger S, Siniard AL, Balak C, et al. Neonatal epileptic encephalopathy caused by de novo GNAO1 mutation misdiagnosed as atypical Rett syndrome: Cautions in interpretation of genomic test results. Semin Pediatr Neurol. 2018;26:28–32.
Du M, Feng J, Tao Y, Pan Q, Chen F. GNAO1 as a Novel Predictive Biomarker for Late Relapse in Hepatocellular Carcinoma. J Health Eng. 2021;2021:7631815.
Song L, Yu B, Yang Y, Liang J, Zhang Y, Ding L, et al. Identification of functional cooperative mutations of GNAO1 in human acute lymphoblastic leukemia. Blood. 2021;137:1181–91.
Takenaka K, Kanaho Y, Hara A, Zhang W, Ando T, Sakai N, et al. A guanine nucleotide-binding protein in human astrocytoma. Neurol Res. 1990;12:223–5.
Zupancic K, Blejec A, Herman A, Veber M, Verbovsek U, Korsic M, et al. Identification of plasma biomarker candidates in glioblastoma using an antibody-array-based proteomic approach. Radio Oncol. 2014;48:257–66.
Wang H, Zhao P, Zhang Y, Chen Z, Bao H, Qian W, et al. NeuroD4 converts glioblastoma cells into neuron-like cells through the SLC7A11-GSH-GPX4 antioxidant axis. Cell Death Discov. 2023;9:297.
Wang K, Pan S, Zhao P, Liu L, Chen Z, Bao H, et al. PTBP1 knockdown promotes neural differentiation of glioblastoma cells through UNC5B receptor. Theranostics. 2022;12:3847–61.
Hatakeyama J, Bessho Y, Katoh K, Ookawara S, Fujioka M, Guillemot F, et al. Hes genes regulate size, shape and histogenesis of the nervous system by control of the timing of neural stem cell differentiation. Development. 2004;131:5539–50.
Ochi S, Imaizumi Y, Shimojo H, Miyachi H, Kageyama R. Oscillatory expression of Hes1 regulates cell proliferation and neuronal differentiation in the embryonic brain. Development. 2020;147:dev182204.
Liu XF, Tang CX, Zhang L, Tong SY, Wang Y, Abdulrahman AA, et al. Down-Regulated CUEDC2 Increases GDNF Expression by Stabilizing CREB Through Reducing Its Ubiquitination in Glioma. Neurochem Res. 2020;45:2915–25.
Chrivia JC, Kwok RP, Lamb N, Hagiwara M, Montminy MR, Goodman RH. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–9.
Wada K, Kamitani T. Autoantigen Ro52 is an E3 ubiquitin ligase. Biochem Bioph Res Co. 2006;339:415–21.
Zhang Z, Bao M, Lu N, Weng L, Yuan B, Liu YJ. The E3 ubiquitin ligase TRIM21 negatively regulates the innate immune response to intracellular double-stranded DNA. Nat Immunol. 2013;14:172–8.
Park JS, Burckhardt CJ, Lazcano R, Solis LM, Isogai T, Li L, et al. Mechanical regulation of glycolysis via cytoskeleton architecture. Nature. 2020;578:621–6.
Liu Q, Sheng Z, Cheng C, Zheng H, Lanuti M, Liu R, et al. Anesthetic Propofol Promotes Tumor Metastasis in Lungs via GABA(A) R-Dependent TRIM21 Modulation of Src Expression. Adv Sci. 2021;8:e2102079.
Lee JH, Liu R, Li J, Zhang C, Wang Y, Cai Q, et al. Stabilization of phosphofructokinase 1 platelet isoform by AKT promotes tumorigenesis. Nat Commun. 2017;8:949.
Zhang HT, Zeng Q, Wu B, Lu J, Tong KL, Lin J, et al. TRIM21-regulated Annexin A2 plasma membrane trafficking facilitates osteosarcoma cell differentiation through the TFEB-mediated autophagy. Cell Death Dis. 2021;12:21.
Towner RA, Zalles M, Saunders D, Smith N. Novel approaches to combat chemoresistance against glioblastomas. Cancer Drug Resist. 2020;3:686–98.
Guichet PO, Bieche I, Teigell M, Serguera C, Rothhut B, Rigau V, et al. Cell death and neuronal differentiation of glioblastoma stem-like cells induced by neurogenic transcription factors. Glia. 2013;61:225–39.
Jiang M, Bajpayee NS. Molecular mechanisms of go signaling. Neurosignals. 2009;17:23–41.
Li JY, Zhao Y, Gong S, Wang MM, Liu X, He QM, et al. TRIM21 inhibits irradiation-induced mitochondrial DNA release and impairs antitumour immunity in nasopharyngeal carcinoma tumour models. Nat Commun. 2023;14:865.
Liu J, Zhang C, Xu D, Zhang T, Chang CY, Wang J. et al. The ubiquitin ligase TRIM21 regulates mutant p53 accumulation and gain of function in cancer. J Clin Investig. 2023;133:e164354.
Funding
This work was supported in part by the National Natural Science Foundation of China (32371004, 82072896 to HF, 32271007 to YL, 82103657 to BS, 82173356 to RY); Program of Shanghai Academic/Technology Research Leader (21XD1403100) to HF; Shanghai Natural Science Foundation (23ZR1441000 to YL).
Author information
Authors and Affiliations
Contributions
HF, YL and HH designed and supervised the project. BS, GW, GC and YZ performed experiments. BS and HF interpreted and/or reviewed the data and wrote the manuscript. GW, GC, YZ and RY edited the manuscript. All authors contributed to the article and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
All authors give consent for the publication of the manuscript.
Ethics approval and consent to participate
All clinical brain tissue specimens were collected at Ren Ji Hospital, Shanghai Jiao Tong University School of Medicine in accordance with a protocol approved by Shanghai Jiao Tong University Institutional Clinical Care and Use Committee of Renji Hospital (Shanghai, China). The investigators obtained informed written consent from the patients. These specimens were examined and diagnosed by pathologists at Ren Ji Hospital. All animal experiments were conducted under the Institutional Animal Care and Use Committee (IACUC)-approved protocols at Ren Ji Hospital, Shanghai Jiao Tong University School of Medicine following NIH and institutional guidelines. The approval number was RJ2022-0828.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, B., Wang, G., Chen, G. et al. GNAO1 overexpression promotes neural differentiation of glioma stem-like cells and reduces tumorigenicity through TRIM21/CREB/HES1 axis. Oncogene 44, 450–461 (2025). https://doi.org/10.1038/s41388-024-03234-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41388-024-03234-7
This article is cited by
-
Novel MAFG-METTL14-SCD1 axis regulates lipid metabolism mediating choroidal melanoma distant metastasis
Journal of Experimental & Clinical Cancer Research (2025)