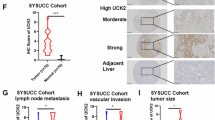
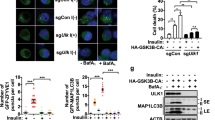
Abstract
Overexpression of uridine-cytidine kinase 2 (UCK2), a key enzyme in the pyrimidine salvage pathway, is implicated in human cancer development, while its regulation under nutrient stress remains to be investigated. Here, we show that under glucose limitation, AMPK phosphorylates glycinamide ribonucleotide formyltransferase (GART) at Ser440, and this modification facilitates its interaction with UCK2. Through its binding to UCK2, GART generates tetrahydrofolate (THF) and thus inhibits the activity of integrin-linked kinase associated phosphatase (ILKAP) for removing AKT1-mediated UCK2-Ser254 phosphorylation under glucose limitation, in which dephosphorylation of UCK2-Ser254 tends to cause Trim21-mediated UCK2 polyubiquitination and degradation. In this way, both UCK2 binding ability and THF producing catalytic activity of GART protect protein stability of UCK2 and pyrimidine salvage synthesis, and sustain tumor cell growth under glucose limitation. In addition, UCK2-Ser254 phosphorylation level displays a positive relationship with GART-Ser440 phosphorylation level and its enhancement is correlated with poor prognosis of human hepatocellular carcinoma (HCC) patients. These findings reveal a non-canonical role of GART in regulating pyrimidine salvage synthesis under nutrient stress, and raise the potential for alternative treatments in targeting pyrimidine salvage-dependent tumor growth.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Supplemental Figures and Table are available in the Supplemental Figures and Table file. The mass spectrometry proteomics data can be accessed at the iProX site.
References
Hotamisligil GS, Davis RJ. Cell signaling and stress responses. Cold Spring Harb Perspect Biol. 2016;8:a006072.
Li X, Egervari G, Wang Y, Berger SL, Lu Z. Regulation of chromatin and gene expression by metabolic enzymes and metabolites. Nat Rev Mol Cell Biol. 2018;19:563–78.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33.
Vander Heiden MG, DeBerardinis RJ. Understanding the intersections between metabolism and cancer biology. Cell. 2017;168:657–69.
Hay N. Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? Nat Rev Cancer. 2016;16:635–49.
Martinez-Reyes I, Chandel NS. Cancer metabolism: looking forward. Nat Rev Cancer. 2021;21:669–80.
O’Malley J, Kumar R, Inigo J, Yadava N, Chandra D. Mitochondrial stress response and cancer. Trends Cancer. 2020;6:688–701.
Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35.
Jones ME. Pyrimidine nucleotide biosynthesis in animals: genes, enzymes, and regulation of UMP biosynthesis. Annu Rev Biochem. 1980;49:253–79.
Traut TW. Physiological concentrations of purines and pyrimidines. Mol Cell Biochem. 1994;140:1–22.
Weber G. Ordered biochemical program of gene expression in cancer cells. Biochemistry (Mosc). 2001;66:1164–73.
Unger MM, Wahl J, Ushmorov A, Buechele B, Simmet T, Debatin KM, et al. Enriching suicide gene bearing tumor cells for an increased bystander effect. Cancer Gene Ther. 2007;14:30–38.
Fairbanks LD, Bofill M, Ruckemann K, Simmonds HA. Importance of ribonucleotide availability to proliferating T-lymphocytes from healthy humans. Disproportionate expansion of pyrimidine pools and contrasting effects of de novo synthesis inhibitors. J Biol Chem. 1995;270:29682–9.
Okesli-Armlovich A, Gupta A, Jimenez M, Auld D, Liu Q, Bassik MC, et al. Discovery of small molecule inhibitors of human uridine-cytidine kinase 2 by high-throughput screening. Bioorg Med Chem Lett. 2019;29:2559–64.
Vander Heiden MG. Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov. 2011;10:671–84.
Spratlin J, Sangha R, Glubrecht D, Dabbagh L, Young JD, Dumontet C, et al. The absence of human equilibrative nucleoside transporter 1 is associated with reduced survival in patients with gemcitabine-treated pancreas adenocarcinoma. Clin Cancer Res. 2004;10:6956–61.
Gaidano V, Houshmand M, Vitale N, Carra G, Morotti A, Tenace V, et al. The Synergism between DHODH Inhibitors and Dipyridamole Leads to Metabolic Lethality in Acute Myeloid Leukemia. Cancers (Basel). 2021;13:1003.
Peters GJ, Kraal I, Pinedo HM. In vitro and in vivo studies on the combination of Brequinar sodium (DUP-785; NSC 368390) with 5-fluorouracil; effects of uridine. Br J Cancer. 1992;65:229–33.
Cysyk RL, Malinowski N, Marquez V, Zaharevitz D, August EM, Moyer JD. Cyclopentenyl uracil: an effective inhibitor of uridine salvage in vivo. Biochem Pharmacol. 1995;49:203–7.
Lane AN, Fan TW. Regulation of mammalian nucleotide metabolism and biosynthesis. Nucleic Acids Res. 2015;43:2466–85.
Van Rompay AR, Norda A, Linden K, Johansson M, Karlsson A. Phosphorylation of uridine and cytidine nucleoside analogs by two human uridine-cytidine kinases. Mol Pharmacol. 2001;59:1181–6.
Zhou Q, Jiang H, Zhang J, Yu W, Zhou Z, Huang P, et al. Uridine-cytidine kinase 2 promotes metastasis of hepatocellular carcinoma cells via the Stat3 pathway. Cancer Manag Res. 2018;10:6339–55.
Cai J, Sun X, Guo H, Qu X, Huang H, Yu C, et al. Non-metabolic role of UCK2 links EGFR-AKT pathway activation to metastasis enhancement in hepatocellular carcinoma. Oncogenesis. 2020;9:103.
Cong X, Lu C, Huang X, Yang D, Cui X, Cai J, et al. Increased expression of glycinamide ribonucleotide transformylase is associated with a poor prognosis in hepatocellular carcinoma, and it promotes liver cancer cell proliferation. Hum Pathol. 2014;45:1370–8.
Liu X, Ding Z, Liu Y, Zhang J, Liu F, Wang X, et al. Glycinamide ribonucleotide formyl transferase is frequently overexpressed in glioma and critically regulates the proliferation of glioma cells. Pathol Res Pract. 2014;210:256–63.
Zhang JM, Jiang C, Ye W, Luo R, Chen HF. Allosteric pathways in tetrahydrofolate sensing riboswitch with dynamics correlation network. Mol Biosyst. 2016;13:156–64.
Loong JH, Wong TL, Tong M, Sharma R, Zhou L, Ng KY, et al. Glucose deprivation-induced aberrant FUT1-mediated fucosylation drives cancer stemness in hepatocellular carcinoma. J Clin Invest. 2021;131:e143377.
Huang Y, Li SR, Gao YJ, Zhu YH, Zhang XF. Novel gene signatures promote epithelial-mesenchymal transition (EMT) in glucose deprivation-Based microenvironment to predict recurrence-free survival in hepatocellular carcinoma. J Oncol. 2023;2023. 6114976.
Xiang J, Chen C, Liu R, Gou D, Chang L, Deng H, et al. Gluconeogenic enzyme PCK1 deficiency promotes CHK2 O-GlcNAcylation and hepatocellular carcinoma growth upon glucose deprivation. J Clin Invest. 2021;131:e144703.
Nwosu ZC, Ward MH, Sajjakulnukit P, Poudel P, Ragulan C, Kasperek S, et al. Uridine-derived ribose fuels glucose-restricted pancreatic cancer. Nature. 2023;618:151–8.
Leung-Hagesteijn C, Mahendra A, Naruszewicz I, Hannigan GE. Modulation of integrin signal transduction by ILKAP, a protein phosphatase 2C associating with the integrin-linked kinase, ILK1. EMBO J. 2001;20:2160–70.
Manieri W, Moore ME, Soellner MB, Tsang P, Caperelli CA. Human glycinamide ribonucleotide transformylase: active site mutants as mechanistic probes. Biochemistry. 2007;46:156–63.
Knox AJ, Graham C, Bleskan J, Brodsky G, Patterson D. Mutations in the Chinese hamster ovary cell GART gene of de novo purine synthesis. Gene. 2009;429:23–30.
Qian X, Li X, Tan L, Lee JH, Xia Y, Cai Q, et al. Conversion of PRPS Hexamer to Monomer by AMPK-Mediated Phosphorylation Inhibits Nucleotide Synthesis in Response to Energy Stress. Cancer Discov. 2018;8:94–107.
Welin M, Grossmann JG, Flodin S, Nyman T, Stenmark P, Tresaugues L, et al. Structural studies of tri-functional human GART. Nucleic Acids Res. 2010;38:7308–19.
Field MS, Kamynina E, Chon J, Stover PJ. Nuclear folate metabolism. Annu Rev Nutr. 2018;38:219–43.
Noguchi H, Prem veer Reddy G, Pardee AB. Rapid incorporation of label from ribonucleoside disphosphates into DNA by a cell-free high molecular weight fraction from animal cell nuclei. Cell. 1983;32:443–51.
Prem veer Reddy G, Pardee AB. Multienzyme complex for metabolic channeling in mammalian DNA replication. Proc Natl Acad Sci USA. 1980;77:3312–6.
Butler P, Pascheto I, Lizzi M, St-Onge R, Lanner C, Guo B, et al. RNA disruption is a widespread phenomenon associated with stress-induced cell death in tumour cells. Sci Rep. 2023;13:1711.
Chen L, Ducker GS, Lu W, Teng X, Rabinowitz JD. An LC-MS chemical derivatization method for the measurement of five different one-carbon states of cellular tetrahydrofolate. Anal Bioanal Chem. 2017;409:5955–64.
Zhao Q, Zheng K, Ma C, Li J, Zhuo L, Huang W, et al. PTPS facilitates compartmentalized LTBP1 S-nitrosylation and promotes tumor Growth under hypoxia. Mol Cell. 2020;77:95–107 e105.
Ma J, Chen T, Wu S, Yang C, Bai M, Shu K, et al. iProX: an integrated proteome resource. Nucleic Acids Res. 2019;47:D1211–D1217.
Chen T, Ma J, Liu Y, Chen Z, Xiao N, Lu Y, et al. iProX in 2021: connecting proteomics data sharing with big data. Nucleic Acids Res. 2022;50:D1522–D1527.
Wang T, Yu Q, Li J, Hu B, Zhao Q, Ma C, et al. O-GlcNAcylation of fumarase maintains tumour growth under glucose deficiency. Nat Cell Biol. 2017;19:833–43.
Lee JH, Liu R, Li J, Wang Y, Tan L, Li XJ, et al. EGFR-Phosphorylated Platelet Isoform of Phosphofructokinase 1 Promotes PI3K Activation. Mol Cell. 2018;70:197–210 e197.
Jakkula P, Qureshi R, Iqbal A, Sagurthi SR, Qureshi IA. Leishmania donovani PP2C: Kinetics, structural attributes and in vitro immune response. Mol Biochem Parasitol. 2018;223:37–49.
Ben-Sahra I, Howell JJ, Asara JM, Manning BD. Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science. 2013;339:1323–8.
Acknowledgements
We thank the staff members of the Large-scale Protein Preparation System at the National Facility for Protein Science in Shanghai (NFPS), Shanghai Advanced Research Institute, Chinese Academy of Sciences, China for providing technical support and assistance in data collection and analysis.
Funding
This work was supported by National Key R&D Program of China (2023YFA1802004 to Y. Jiang), National Nature Science Foundation of China (82273230 to Q. Zhao; 81972586 to Y. Jiang).
Author information
Authors and Affiliations
Contributions
This study was conceived by Q. Zhao, Y. Jiang and Q. Xia; Q. Zhao and Y. Jiang designed the study; Q. Zhao, N. Sha, B. Zhou, G. Hou, Z. Xi, W. Wang, M. Yan, J. He, Y. Zhou performed experiments; Q. Zhao and Y. Jiang wrote the paper with comments from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The authors confirm that all methods were performed in accordance with the relevant guidelines and regulations. This study was reviewed and approved by Shanghai Jiaotong University School of Medicine, Renji Hospital Ethics Committee (KY2024-152-B) with written informed consents from all the patients.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sha, N., Zhou, B., Hou, G. et al. The protection of UCK2 protein stability by GART maintains pyrimidine salvage synthesis for HCC growth under glucose limitation. Oncogene 44, 1078–1092 (2025). https://doi.org/10.1038/s41388-025-03274-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41388-025-03274-7