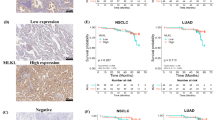
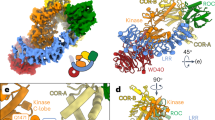
Abstract
Tyrosine kinase inhibitor (TKI) and immune checkpoint inhibitor (ICI) combination therapy is emerging as a major therapeutic strategy for advanced clear cell renal cell carcinoma (ccRCC). To define the druggable targets for improvement of TKI and ICI combination therapy in ccRCC, we analyzed a commercial protein kinase inhibitor dataset and a public ccRCC dataset and identified LRRK2 as a potential candidate that can be targeted by a small molecule inhibitor. We demonstrated that LRRK2 was transcriptionally upregulated by HIF2A and enabled to drive proliferation of ccRCC cells in a manner independent of its kinase activity. LRRK2 inhibits the RBX1-mediated degradation of lipid metabolism modulator LPCAT1 to reducing the sensitivity to TKI and PD-1 blockade in ccRCC. Specifically, LRRK2/LPCAT1 upregulated IL-1β expression levels through AKT and also increased IL-1β shearing by activating inflammasome. To target the kinase-independent activity of LRRK2, we developed an LR-protac and showed that LR-protac decreased LRRK2 protein level and enhanced the antitumor effect of PD-1 blockade and TKI in ccRCC. These data indicate that LRRK2 is a viable target for improvement of the efficacy of PD-1 blockade and TKI in ccRCC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable request. The data generated in this study are publicly available in Gene Expression Omnibus (GEO) at GSE264320.
References
Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol. 2016;70:93–105.
Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12–49.
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48.
Tran J, Ornstein MC. Clinical Review on the Management of Metastatic Renal Cell Carcinoma. JCO Oncol Pract. 2022;18:187–96.
Ljungberg B, Albiges L, Abu-Ghanem Y, Bensalah K, Dabestani S, Fernandez-Pello S, et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2019 Update. Eur Urol. 2019;75:799–810.
Shenoy N, Pagliaro L. Sequential pathogenesis of metastatic VHL mutant clear cell renal cell carcinoma: putting it together with a translational perspective. Ann Oncol. 2016;27:1685–95.
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, et al. Overall Survival and Updated Results for Sunitinib Compared With Interferon Alfa in Patients With Metastatic Renal Cell Carcinoma. J Clin Oncol. 2023;41:1965–71.
Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931–9.
Motzer RJ, Hutson TE, Ren M, Dutcus C, Larkin J. Independent assessment of lenvatinib plus everolimus in patients with metastatic renal cell carcinoma. Lancet Oncol. 2016;17:e4–5.
Teisseire M, Giuliano S, Pages G. Combination of Anti-Angiogenics and Immunotherapies in Renal Cell Carcinoma Show Their Limits: Targeting Fibrosis to Break through the Glass Ceiling? Biomedicines. 2024;12:385.
Liu XD, Hoang A, Zhou L, Kalra S, Yetil A, Sun M, et al. Resistance to Antiangiogenic Therapy Is Associated with an Immunosuppressive Tumor Microenvironment in Metastatic Renal Cell Carcinoma. Cancer Immunol Res. 2015;3:1017–29.
Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2019;380:1116–27.
Cella D, Motzer RJ, Suarez C, Blum SI, Ejzykowicz F, Hamilton M, et al. Patient-reported outcomes with first-line nivolumab plus cabozantinib versus sunitinib in patients with advanced renal cell carcinoma treated in CheckMate 9ER: an open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23:292–303.
Motzer R, Alekseev B, Rha SY, Porta C, Eto M, Powles T, et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N Engl J Med. 2021;384:1289–300.
Vano YA, Elaidi R, Bennamoun M, Chevreau C, Borchiellini D, Pannier D, et al. Nivolumab, nivolumab-ipilimumab, and VEGFR-tyrosine kinase inhibitors as first-line treatment for metastatic clear-cell renal cell carcinoma (BIONIKK): a biomarker-driven, open-label, non-comparative, randomised, phase 2 trial. Lancet Oncol. 2022;23:612–24.
Wang Y, Peng M, Zhong Y, Xiong W, Zhu L, Jin X. The E3 ligase RBCK1 reduces the sensitivity of ccRCC to sunitinib through the ANKRD35-MITD1-ANXA1 axis. Oncogene. 2023;42:952–66.
Liu W, Wang H, Jian C, Li W, Ye K, Ren J, et al. The RNF26/CBX7 axis modulates the TNF pathway to promote cell proliferation and regulate sensitivity to TKIs in ccRCC. Int J Biol Sci. 2022;18:2132–45.
Liu W, Ren D, Xiong W, Jin X, Zhu L. A novel FBW7/NFAT1 axis regulates cancer immunity in sunitinib-resistant renal cancer by inducing PD-L1 expression. J Exp Clin Cancer Res. 2022;41:38.
Liu W, Yan B, Yu H, Ren J, Peng M, Zhu L, et al. OTUD1 stabilizes PTEN to inhibit the PI3K/AKT and TNF-alpha/NF-kappaB signaling pathways and sensitize ccRCC to TKIs. Int J Biol Sci. 2022;18:1401–14.
Xiong W, Zhang B, Yu H, Zhu L, Yi L, Jin X. RRM2 Regulates Sensitivity to Sunitinib and PD-1 Blockade in Renal Cancer by Stabilizing ANXA1 and Activating the AKT Pathway. Adv Sci (Weinh). 2021;8:e2100881.
Chiang IC, Chen SY, Hsu YH, Shahidi F, Yen GC. Pterostilbene and 6-shogaol exhibit inhibitory effects on sunitinib resistance and motility by suppressing the RLIP76-initiated Ras/ERK and Akt/mTOR pathways in renal cancer cells. Eur J Pharmacol. 2024;967:176393.
Zerdes I, Matikas A, Bergh J, Rassidakis GZ, Foukakis T. Genetic, transcriptional and post-translational regulation of the programmed death protein ligand 1 in cancer: biology and clinical correlations. Oncogene. 2018;37:4639–61.
Wang H, Wang H, Chen J, Liu P, Xiao X. Overexpressed FAM111B degrades GSDMA to promote esophageal cancer tumorigenesis and cisplatin resistance. Cell Oncol (Dordr). 2024;47:343–59.
Miikkulainen P, Hogel H, Seyednasrollah F, Rantanen K, Elo LL, Jaakkola PM. Hypoxia-inducible factor (HIF)-prolyl hydroxylase 3 (PHD3) maintains high HIF2A mRNA levels in clear cell renal cell carcinoma. J Biol Chem. 2019;294:3760–71.
Mathew LK, Lee SS, Skuli N, Rao S, Keith B, Nathanson KL, et al. Restricted expression of miR-30c-2-3p and miR-30a-3p in clear cell renal cell carcinomas enhances HIF2alpha activity. Cancer Discov. 2014;4:53–60.
Hoefflin R, Harlander S, Schafer S, Metzger P, Kuo F, Schonenberger D, et al. HIF-1alpha and HIF-2alpha differently regulate tumour development and inflammation of clear cell renal cell carcinoma in mice. Nat Commun. 2020;11:4111.
Liu H, Ho PW, Leung CT, Pang SY, Chang EES, Choi ZY, et al. Aberrant mitochondrial morphology and function associated with impaired mitophagy and DNM1L-MAPK/ERK signaling are found in aged mutant Parkinsonian LRRK2(R1441G) mice. Autophagy. 2021;17:3196–220.
Qu YY, Zhao R, Zhang HL, Zhou Q, Xu FJ, Zhang X, et al. Inactivation of the AMPK-GATA3-ECHS1 Pathway Induces Fatty Acid Synthesis That Promotes Clear Cell Renal Cell Carcinoma Growth. Cancer Res. 2020;80:319–33.
Tao M, Luo J, Gu T, Yu X, Song Z, Jun Y, et al. LPCAT1 reprogramming cholesterol metabolism promotes the progression of esophageal squamous cell carcinoma. Cell Death Dis. 2021;12:845.
Du Y, Wang Q, Zhang X, Wang X, Qin C, Sheng Z, et al. Lysophosphatidylcholine acyltransferase 1 upregulation and concomitant phospholipid alterations in clear cell renal cell carcinoma. J Exp Clin Cancer Res. 2017;36:66.
Ding J, Ding X, Leng Z. LPCAT1 promotes gefitinib resistance via upregulation of the EGFR/PI3K/AKT signaling pathway in lung adenocarcinoma. J Cancer. 2022;13:1837–47.
Wang Y, Huang Y, Wang Y, Zhang W, Wang N, Bai R, et al. LPCAT1 promotes melanoma cell proliferation via Akt signaling. Oncol Rep. 2024;51:67.
Wei C, Dong X, Lu H, Tong F, Chen L, Zhang R, et al. LPCAT1 promotes brain metastasis of lung adenocarcinoma by up-regulating PI3K/AKT/MYC pathway. J Exp Clin Cancer Res. 2019;38:95.
Dell’Atti L, Bianchi N, Aguiari G. New Therapeutic Interventions for Kidney Carcinoma: Looking to the Future. Cancers (Basel). 2022;14:3616.
Prevete N, Liotti F, Amoresano A, Pucci P, de Paulis A, Melillo RM. New perspectives in cancer: Modulation of lipid metabolism and inflammation resolution. Pharmacol Res. 2018;128:80–7.
Lai Y, Tang F, Huang Y, He C, Chen C, Zhao J, et al. The tumour microenvironment and metabolism in renal cell carcinoma targeted or immune therapy. J Cell Physiol. 2021;236:1616–27.
Zhao X, Lian X, Xie J, Liu G. Accumulated cholesterol protects tumours from elevated lipid peroxidation in the microenvironment. Redox Biol. 2023;62:102678.
Aggen DH, Ager CR, Obradovic AZ, Chowdhury N, Ghasemzadeh A, Mao W, et al. Blocking IL1 Beta Promotes Tumor Regression and Remodeling of the Myeloid Compartment in a Renal Cell Carcinoma Model: Multidimensional Analyses. Clin Cancer Res. 2021;27:608–21.
Huang Y, Wang Y, Zhen Y, Liu W, Wang Y, Wang R, et al. LPCAT1 Facilitates Keratinocyte Hyperproliferation and Skin Inflammation in Psoriasis by Regulating GLUT3. J Invest Dermatol. 2024;144:1479–49.e14.
Wang Y, Che M, Xin J, Zheng Z, Li J, Zhang S. The role of IL-1beta and TNF-alpha in intervertebral disc degeneration. Biomed Pharmacother. 2020;131:110660.
Yuan B, Clowers MJ, Velasco WV, Peng S, Peng Q, Shi Y, et al. Targeting IL-1beta as an immunopreventive and therapeutic modality for K-ras-mutant lung cancer. JCI Insight. 2022;7:e157788.
Tian T, Lofftus S, Pan Y, Stingley CA, King SL, Zhao J, et al. IL1alpha Antagonizes IL1beta and Promotes Adaptive Immune Rejection of Malignant Tumors. Cancer Immunol Res. 2020;8:660–71.
Yap TA, Yan L, Patnaik A, Fearen I, Olmos D, Papadopoulos K, et al. First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin Oncol. 2011;29:4688–95.
Ling YJ, Ding TY, Dong FL, Gao YJ, Jiang BC. Intravenous Administration of Triptonide Attenuates CFA-Induced Pain Hypersensitivity by Inhibiting DRG AKT Signaling Pathway in Mice. J Pain Res. 2020;13:3195–206.
Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–32.
Wang Y, Gao JZ, Sakaguchi T, Maretzky T, Gurung P, Narayanan NS, et al. LRRK2 G2019S Promotes Colon Cancer Potentially via LRRK2-GSDMD Axis-Mediated Gut Inflammation. Cells. 2024;13:565.
Soupene E, Fyrst H, Kuypers FA. Mammalian acyl-CoA:lysophosphatidylcholine acyltransferase enzymes. Proc Natl Acad Sci USA. 2008;105:88–93.
Moessinger C, Kuerschner L, Spandl J, Shevchenko A, Thiele C. Human lysophosphatidylcholine acyltransferases 1 and 2 are located in lipid droplets where they catalyze the formation of phosphatidylcholine. J Biol Chem. 2011;286:21330–9.
Han YH, Liu XD, Jin MH, Sun HN, Kwon T. Role of NLRP3 inflammasome-mediated neuronal pyroptosis and neuroinflammation in neurodegenerative diseases. Inflamm Res. 2023;72:1839–59.
Sun Y, Zhu L, Liu P, Zhang H, Guo F, Jin X. ZDHHC2-Mediated AGK Palmitoylation Activates AKT-mTOR Signaling to Reduce Sunitinib Sensitivity in Renal Cell Carcinoma. Cancer Res. 2023;83:2034–51.
Rutherford KA, McManus KJ. PROTACs: Current and Future Potential as a Precision Medicine Strategy to Combat Cancer. Mol Cancer Ther. 2024;23:454–63.
Choueiri TK, Eto M, Motzer R, De Giorgi U, Buchler T, Basappa NS, et al. Lenvatinib plus pembrolizumab versus sunitinib as first-line treatment of patients with advanced renal cell carcinoma (CLEAR): extended follow-up from the phase 3, randomised, open-label study. Lancet Oncol. 2023;24:228–38.
Kitajima S, Tani T, Springer BF, Campisi M, Osaki T, Haratani K, et al. MPS1 inhibition primes immunogenicity of KRAS-LKB1 mutant lung cancer. Cancer Cell. 2022;40:1128–44 e1128.
Wang M, Zhu L, Yang X, Li J, Liu Y, Tang Y. Targeting immune cell types of tumor microenvironment to overcome resistance to PD-1/PD-L1 blockade in lung cancer. Front Pharmacol. 2023;14:1132158.
Jin H, Shi Y, Lv Y, Yuan S, Ramirez CFA, Lieftink C, et al. EGFR activation limits the response of liver cancer to lenvatinib. Nature. 2021;595:730–4.
Wojewska DN, Kortholt A. LRRK2 Targeting Strategies as Potential Treatment of Parkinson’s Disease. Biomolecules. 2021;11:1101.
Sosero YL, Gan-Or Z. LRRK2 and Parkinson’s disease: from genetics to targeted therapy. Ann Clin Transl Neurol. 2023;10:850–64.
Jennings D, Huntwork-Rodriguez S, Vissers M, Daryani VM, Diaz D, Goo MS, et al. LRRK2 Inhibition by BIIB122 in Healthy Participants and Patients with Parkinson’s Disease. Mov Disord. 2023;38:386–98.
Qiu Y, Liu L, Yang H, Chen H, Deng Q, Xiao D, et al. Integrating Histologic and Genomic Characteristics to Predict Tumor Mutation Burden of Early-Stage Non-Small-Cell Lung Cancer. Front Oncol. 2020;10:608989.
Cheung M, Kadariya Y, Sementino E, Hall MJ, Cozzi I, Ascoli V, et al. Novel LRRK2 mutations and other rare, non-BAP1-related candidate tumor predisposition gene variants in high-risk cancer families with mesothelioma and other tumors. Hum Mol Genet. 2021;30:1750–61.
Wu YM, Sa Y, Guo Y, Li QF, Zhang N. Identification of WHO II/III Gliomas by 16 Prognostic-related Gene Signatures using Machine Learning Methods. Curr Med Chem. 2022;29:1622–39.
Yang C, Pang J, Xu J, Pan H, Li Y, Zhang H, et al. LRRK2 is a candidate prognostic biomarker for clear cell renal cell carcinoma. Cancer Cell Int. 2021;21:343.
Park S, Kim KH, Bae YH, Oh YT, Shin H, Kwon HJ, et al. Suppression of Glioblastoma Stem Cell Potency and Tumor Growth via LRRK2 Inhibition. Int J Stem Cells. 2024;17:319–29.
Zhou L, Luo Y, Liu Y, Zeng Y, Tong J, Li M, et al. Fatty Acid Oxidation Mediated by Malonyl-CoA Decarboxylase Represses Renal Cell Carcinoma Progression. Cancer Res. 2023;83:3920–39.
Yao D, Xia S, Jin C, Zhao W, Lan W, Liu Z, et al. Feedback activation of GATA1/miR-885-5p/PLIN3 pathway decreases sunitinib sensitivity in clear cell renal cell carcinoma. Cell Cycle. 2020;19:2195–206.
Jiang H, Li Z, Huan C, Jiang XC. Macrophage Lysophosphatidylcholine Acyltransferase 3 Deficiency-Mediated Inflammation Is Not Sufficient to Induce Atherosclerosis in a Mouse Model. Front Cardiovasc Med. 2018;5:192.
Wang B, Tontonoz P. Phospholipid Remodeling in Physiology and Disease. Annu Rev Physiol. 2019;81:165–88.
Bent R, Moll L, Grabbe S, Bros M. Interleukin-1 Beta-A Friend or Foe in Malignancies? Int J Mol Sci. 2018;19:2155.
Acknowledgements
We would like to thank Shanghai BioProfile Biotechnology (Shanghai, China) for technical support. We would like to thank ProMab Biotechnologies, Inc. (Changsha, China) for their antibody.
Author information
Authors and Affiliations
Contributions
Yulong Hong: Methodology; Wei Li: Methodology; Zhuo Xing: Methodology; Minghao Lu: Methodology; Tianyu Tang: Formal analysis; Liang Zhu: Formal analysis; Wei Xiong: Formal analysis; Wentao Liu: Project administration, Investigation; Huan Zhang: Investigation; Shangqing Ren: Project administration, Investigation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
We confirm that all methods are performed in accordance with the relevant guidelines and regulations. The study was approved by the Animal Use and Care Committees at the Second Xiangya hospital, Central South University (License No. 20240511). We obtained informed consent from all patients to whom the specimens belonged. We obtained informed consent from all patients for the use of their specimens.
Consent for publication
All subjects have written informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hong, Y., Li, W., Xing, Z. et al. LRRK2 reduces the sensitivity to TKI and PD-1 blockade in ccRCC via activating LPCAT1. Oncogene 44, 1761–1776 (2025). https://doi.org/10.1038/s41388-025-03289-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41388-025-03289-0
This article is cited by
-
Gut microbiota drives cancer evolution and therapy resistance
Molecular Cancer (2026)