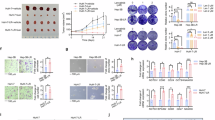
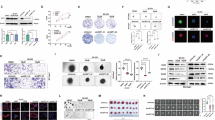
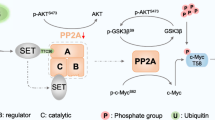
Abstract
Hepatocellular carcinoma (HCC) is a major global health burden, with metabolic dysfunction-associated steatohepatitis (MASH) emerging as a significant risk factor. The scarcity of effective pharmacological treatments for MASH and its progression to HCC underscores the need for deeper molecular insights. Our study identifies Myc-binding protein 2 (MYCBP2), an E3 ubiquitin ligase, as a potential tumor suppressor in MASH-related HCC. Through transcriptomic and proteomic analyses, we observed significant downregulation of MYCBP2 in HCC tissues. In vitro and in vivo experiments demonstrate that MYCBP2 inhibits HCC cell proliferation, migration, and invasion by modulating lipid metabolism pathways. Mechanistically, MYCBP2 promotes the ubiquitination and degradation of Hepatocyte Nuclear Factor 4 Alpha (HNF4α). This ubiquitination occurs via K33- and K48-linked polyubiquitin chains at lysines 300 and 307 of HNF4α. The results showed that MYCBP2 influences the expression of lipid metabolism-related genes and attenuates HNF4α‘s regulatory role in lipid metabolism through the mediated ubiquitination and degradation of HNF4α. Our findings elucidate the MYCBP2-HNF4α axis as a novel regulatory pathway in MASH-related HCC and highlight the broader implications of ubiquitination in cancer metabolism, offering a promising metabolic target for therapeutic intervention.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are included in this published article and its supplementary materials. Additional data supporting the findings of this study are available on Figshare (Figshare https://doi.org/10.6084/m9.figshare.27758889).
References
Toh MR, Wong EYT, Wong SH, Ng AWT, Loo LH, Chow PK, et al. Global epidemiology and genetics of hepatocellular carcinoma. Gastroenterology. 2023;164:766–82.
Shah PA, Patil R, Harrison SA. NAFLD-related hepatocellular carcinoma: The growing challenge. Hepatology. 2023;77:323–38.
Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79:1542–56.
Ha S, Wong VW, Zhang X, Yu J. Interplay between gut microbiome, host genetic and epigenetic modifications in MASLD and MASLD-related hepatocellular carcinoma. Gut. 2024.
Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18:223–38.
Younossi ZM, Henry L. Epidemiology of non-alcoholic fatty liver disease and hepatocellular carcinoma. JHEP Rep. 2021;3. 100305.
Apostolo D, Ferreira LL, Vincenzi F, Vercellino N, Minisini R, Latini F, et al. From MASH to HCC: the role of Gas6/TAM receptors. Front Immunol. 2024;15. 1332818.
Cheng L, Deepak R, Wang G, Meng Z, Tao L, Xie M, et al. Hepatic mitochondrial NAD + transporter SLC25A47 activates AMPKα mediating lipid metabolism and tumorigenesis. Hepatology. 2023;78:1828–42.
Ding Z, Pan Y, Shang T, Jiang T, Lin Y, Yang C, et al. URI alleviates tyrosine kinase inhibitors-induced ferroptosis by reprogramming lipid metabolism in p53 wild-type liver cancers. Nat Commun. 2023;14. 6269.
Hu J, Wang H, Li X, Liu Y, Mi Y, Kong H, et al. Fibrinogen-like protein 2 aggravates nonalcoholic steatohepatitis via interaction with TLR4, eliciting inflammation in macrophages and inducing hepatic lipid metabolism disorder. Theranostics. 2020;10:9702–20.
Park J, Zhao Y, Zhang F, Zhang S, Kwong AC, Zhang Y, et al. IL-6/STAT3 axis dictates the PNPLA3-mediated susceptibility to non-alcoholic fatty liver disease. J Hepatol. 2023;78:45–56.
Zhai X, Xia Z, Du G, Zhang X, Xia T, Ma D, et al. LRP1B suppresses HCC progression through the NCSTN/PI3K/AKT signaling axis and affects doxorubicin resistance. Genes Dis. 2023;10:2082–96.
Kim YS, Lee YM, Oh TI, Shin DH, Kim GH, Kan SY, et al. Emodin sensitizes hepatocellular carcinoma cells to the anti-cancer effect of sorafenib through suppression of cholesterol metabolism. Int J Mol Sci. 2018;19:3127.
AlAbdi L, Desbois M, Rusnac DV, Sulaiman RA, Rosenfeld JA, Lalani S, et al. Loss-of-function variants in MYCBP2 cause neurobehavioural phenotypes and corpus callosum defects. Brain. 2023;146:1373–87.
Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell. 2006;126:789–99.
Cheng W, Li X, Zhou Y, Yu H, Xie Y, Guo H, et al. Polystyrene microplastics induce hepatotoxicity and disrupt lipid metabolism in the liver organoids. Science Total Environ. 2022;806. 150328.
Yang T, Poenisch M, Khanal R, Hu Q, Dai Z, Li R, et al. Therapeutic HNF4A mRNA attenuates liver fibrosis in a preclinical model. J Hepatol. 2021;75:1420–33.
Foerster F, Gairing SJ, Müller L, Galle PR. NAFLD-driven HCC: Safety and efficacy of current and emerging treatment options. J Hepatol. 2022;76:446–57.
Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20.
Llovet JM, Willoughby CE, Singal AG, Greten TF, Heikenwälder M, El-Serag HB, et al. Nonalcoholic steatohepatitis-related hepatocellular carcinoma: pathogenesis and treatment. Nat Rev Gastroenterol Hepatol. 2023;20:487–503.
Wei W, Wong CC, Jia Z, Liu W, Liu C, Ji F, et al. Parabacteroides distasonis uses dietary inulin to suppress NASH via its metabolite pentadecanoic acid. Nat Microbiol. 2023;8:1534–48.
Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908–22.
Jin K, Shi Y, Zhang H, Zhangyuan G, Wang F, Li S, et al. A TNFα/Miz1-positive feedback loop inhibits mitophagy in hepatocytes and propagates non-alcoholic steatohepatitis. J Hepatol. 2023;79:403–16.
Li K, Zhang K, Wang H, Wu Y, Chen N, Chen J, et al. Hrd1-mediated ACLY ubiquitination alleviate NAFLD in db/db mice. Metab Clin Exp. 2021;114. 154349.
Loix M, Zelcer N, Bogie JFJ, Hendriks JJA. The ubiquitous role of ubiquitination in lipid metabolism. Trends Cell Biol. 2024;34:416–29.
Liu F, Chen J, Li K, Li H, Zhu Y, Zhai Y, et al. Ubiquitination and deubiquitination in cancer: from mechanisms to novel therapeutic approaches. Mol Cancer. 2024;23. 148.
Cockram PE, Kist M, Prakash S, Chen SH, Wertz IE, Vucic D. Ubiquitination in the regulation of inflammatory cell death and cancer. Cell Death Differ. 2021;28:591–605.
Zhang H, Xia T, Xia Z, Zhou H, Li Z, Wang W, et al. KIF18A inactivates hepatic stellate cells and alleviates liver fibrosis through the TTC3/Akt/mTOR pathway. Cellular Mol Life Sci. 2024;81:96.
Yang Q, Chen X, Zhang Y, Hu S, Hu F, Huang Y, et al. The E3 ubiquitin ligase ring finger protein 5 ameliorates NASH through ubiquitin-mediated degradation of 3-hydroxy-3-methylglutaryl CoA reductase degradation protein 1. Hepatology. 2021;74:3018–36.
Pi Y, Feng Q, Sun F, Wang Z, Zhao Y, Chen D, et al. Loss of SMURF2 expression enhances RACK1 stability and promotes ovarian cancer progression. Cell Death Differ. 2023;30:2382–92.
Arimoto KI, Miyauchi S, Troutman TD, Zhang Y, Liu M, Stoner SA, et al. Expansion of interferon inducible gene pool via USP18 inhibition promotes cancer cell pyroptosis. Nat. Commun. 2023;14. 251.
Scott K, Hayden PJ, Will A, Wheatley K, Coyne I. Bortezomib for the treatment of multiple myeloma. Cochrane Database Syst Rev. 2016;4:Cd010816.
Ren H, Hu F, Wang D, Kang X, Feng X, Zhang L, et al. Sirtuin 2 Prevents Liver steatosis and metabolic disorders by deacetylation of hepatocyte nuclear factor 4α. Hepatology. 2021;74:723–40.
Seo E, Nam H, Jun HS. Reactive oxygen species induce HNF-4α expression via the ASK1-CREB pathway, promoting ChREBP expression and lipogenesis in hepatocytes. Life Sci. 2022;310: 121042.
Xiao MC, Jiang N, Chen LL, Liu F, Liu SQ, Ding CH, et al. TRIB3-TRIM8 complex drives NAFLD progression by regulating HNF4α stability. J Hepatol. 2024;80:778–91.
Acknowledgement
We would like to express our gratitude to the teachers from the Department of Pathology at the Second Hospital of Shandong University for their assistance in this study. We also thank Freescience for providing editing services for this research.
Funding
This study was funded by the China Postdoctoral Science Foundation (Grant No. 2023M742128); the National Natural Science Foundation of China (Grant No. 82403916); the National Key Research and Development Program of China (Grant No. 2023YFF1204003) and the Shandong Provincial Natural Science Foundation (Grant No. ZR2024QH227).
Author information
Authors and Affiliations
Contributions
HZ, XXK and HRQ conceived and performed most of the experiments; YG, ZYG and ZQY accomplished some of the in vitro experiments. HXZ, KQL and WW analyzed the data. HZ, XYZ and BJ wrote the manuscript. All authors had given approval to the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All methods in this study were performed in accordance with the relevant guidelines and regulations, including the Declaration of Helsinki for human participants and the ARRIVE guidelines for animal experiments. Ethics approval for the research involving live vertebrates (animals) was obtained from the Animal Use and Care Committee of the Second Hospital of Shandong University (License No. KYLL2024857). Ethics approval for the use of human samples and data was granted by the Scientific Research Ethics Committee of the Second Hospital of Shandong University (License No. KYLL2023413). Informed consent was obtained from all human participants prior to their inclusion in the study. No participants under the age of 18 were involved in this research. Additionally, written informed consent for the publication of identifiable images was obtained separately from all participants whose images are included in this manuscript.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, H., Kong, X., Qu, H. et al. MYCBP2-mediated HNF4α ubiquitination reprogrammed lipid metabolism in MASH-associated hepatocellular carcinoma. Oncogene 44, 1961–1974 (2025). https://doi.org/10.1038/s41388-025-03373-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41388-025-03373-5