Abstract
Epithelial ovarian cancer (EOC), the deadliest gynecological malignancy, is increasingly linked to dysregulated lipid metabolism. Nevertheless, the involvement of circulating low-density lipoprotein (LDL) in ovarian cancer progression remains controversial. Analyses of single-cell RNA sequencing and clinical data demonstrated a positive correlation between elevated LDL levels and EOC progression. Mechanistically, LDL internalized via LDL receptor (LDLR) enhanced epithelial-mesenchymal transition (EMT) and stemness in ovarian cancer cells, driven by the upregulation of the key transcription factor FOXQ1. Intriguingly, our investigations unveiled a novel transcriptional complex comprising FOXQ1/β-Catenin/ADNP. Both β-Catenin and ADNP interacted with FOXQ1 at the Forkhead domain, where FOXQ1 bound to the NF-κB1 gene promoter to enhance transcriptional activation. Notably, β-Catenin and ADNP were identified for the first time as competitive repressors within this regulatory axis. These findings were further corroborated in vivo using an ovarian cancer xenograft metastasis model, as well as in human pathological specimens, highlighting LDL-driven metastasis via FOXQ1 upregulation. Collectively, LDL promotes ovarian cancer metastasis through LDLR/FOXQ1/NF-κB1 axis. Furthermore, we discover a novel transcriptional complex, where FOXQ1 acts as the central regulator while β-Catenin/ADNP serve as co-repressors. These insights suggest that modulating serum LDL levels or targeting FOXQ1 may offer promising strategies to curb ovarian cancer progression.

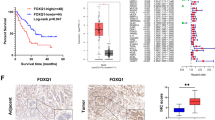
LDL promotes ovarian cancer metastasis through LDLR/FOXQ1/NF-κB1 axis. High serum LDL uptake mediated by LDLR enhances EMT and stemness of ovarian cancer cells via upregulating FOXQ1 expression. Both β-Catenin and ADNP interact with FOXQ1 in the Forkhead domain (FH), also where FOXQ1 binds to NF-κB1 gene promoter to active its transcription, suggesting that β-Catenin and ADNP may act as competitive repressors in this novel transcriptional regulatory complex. Thus, controlling serum LDL levels and targeting FOXQ1 may be effective interventions for preventing metastasis in women with ovarian cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The RNA sequencing data have been uploaded to the GEO database (GSE301160). All the raw data and code generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49.
Buza N. Immunohistochemistry in gynecologic carcinomas: practical update with diagnostic and clinical considerations based on the 2020 WHO classification of tumors. Semin Diagn Pathol. 2022;39:58–77.
Sambasivan S. Epithelial ovarian cancer: review article. Cancer Treat Res Commun. 2022;33:100629.
Webb PM, Jordan SJ. Epidemiology of epithelial ovarian cancer. Best Pr Res Clin Obstet Gynaecol. 2017;41:3–14.
Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–91.
Vekic J, Zeljkovic A, Stefanovic A, Jelic-Ivanovic Z, Spasojevic-Kalimanovska V. Obesity and dyslipidemia. Metabolism. 2019;92:71–81.
Ji Z, Shen Y, Feng X, Kong Y, Shao Y, Meng J, et al. Deregulation of Lipid Metabolism: The Critical Factors in Ovarian Cancer. Front Oncol. 2020;10:593017.
Yarmolinsky J, Bull CJ, Vincent EE, Robinson J, Walther A, Smith GD, et al. Association between genetically proxied inhibition of HMG-CoA reductase and epithelial ovarian cancer. Jama. 2020;323:646–55.
Trabert B, Hathaway CA, Rice MS, Rimm EB, Sluss PM, Terry KL, et al. Ovarian cancer risk in relation to blood cholesterol and triglycerides. Cancer Epidemiol Biomark Prev. 2021;30:2044–51.
Zhang D, Xi Y, Feng Y. Ovarian cancer risk in relation to blood lipid levels and hyperlipidemia: a systematic review and meta-analysis of observational epidemiologic studies. Eur J Cancer Prev. 2021;30:161–70.
Lin Q, Liu W, Xu S, Sun L. Associations of preoperative serum high-density lipoprotein cholesterol and low-density lipoprotein cholesterol levels with the prognosis of ovarian cancer. Arch Gynecol Obstet. 2022;305:683–91.
Li AJ, Elmore RG, Chen IY, Karlan BY. Serum low-density lipoprotein levels correlate with survival in advanced stage epithelial ovarian cancers. Gynecol Oncol. 2010;116:78–81.
Tang S, Zheng F, Chen K, Niu Y, Fu Z, Wu Y, et al. Novel scoring system incorporating lipoproteins to predict outcomes of epithelial ovarian cancer patients. Int J Gynecol Cancer. 2024.
Chang WC, Wang HC, Cheng WC, Yang JC, Chung WM, Ho YP, et al. LDLR-mediated lipidome-transcriptome reprogramming in cisplatin insensitivity. Endocr Relat Cancer. 2020;27:81–95.
Scoles DR, Xu X, Wang H, Tran H, Taylor-Harding B, Li A, et al. Liver X receptor agonist inhibits proliferation of ovarian carcinoma cells stimulated by oxidized low density lipoprotein. Gynecol Oncol. 2010;116:109–16.
Babaei G, Aziz SG, Jaghi NZZ. EMT, cancer stem cells and autophagy; the three main axes of metastasis. Biomed Pharmacother. 2021;133:110909.
Najafi M, Mortezaee K, Majidpoor J. Cancer stem cell (CSC) resistance drivers. Life Sci. 2019;234:116781.
Song Y, Chen Y, Li Y, Lyu X, Cui J, Cheng Y, et al. Resveratrol suppresses epithelial-mesenchymal transition in GBM by regulating smad-dependent signaling. Biomed Res Int. 2019;2019:1321973.
Bieller A, Pasche B, Frank S, Gläser B, Kunz J, Witt K, et al. Isolation and characterization of the human forkhead gene FOXQ1. DNA Cell Biol. 2001;20:555–61.
Li Y, Zhang Y, Yao Z, Li S, Yin Z, Xu M. Forkhead box Q1: a key player in the pathogenesis of tumors (Review). Int J Oncol. 2016;49:51–58.
Wu J, Wu Y, Chen S, Guo Q, Shao Y, Liu C, et al. PARP1-stabilised FOXQ1 promotes ovarian cancer progression by activating the LAMB3/WNT/β-catenin signalling pathway. Oncogene. 2024;43:866–83.
Bassan M, Zamostiano R, Davidson A, Pinhasov A, Giladi E, Perl O, et al. Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. J Neurochem. 1999;72:1283–93.
Blaj C, Bringmann A, Schmidt EM, Urbischek M, Lamprecht S, Fröhlich T, et al. ADNP Is a Therapeutically Inducible Repressor of WNT Signaling in Colorectal Cancer. Clin Cancer Res. 2017;23:2769–80.
Karagoz K, Mehta GA, Khella CA, Khanna P, Gatza ML. Integrative proteogenomic analyses of human tumours identifies ADNP as a novel oncogenic mediator of cell cycle progression in high-grade serous ovarian cancer with poor prognosis. EBioMedicine. 2019;50:191–202.
Ozawa M, Baribault H, Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. Embo j. 1989;8:1711–7.
Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang X, et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther. 2022;7:3.
Tortelote GG, Reis RR, de Almeida Mendes F, Abreu JG. Complexity of the Wnt/β‑catenin pathway: Searching for an activation model. Cell Signal. 2017;40:30–43.
Go GW, Mani A. Low-density lipoprotein receptor (LDLR) family orchestrates cholesterol homeostasis. Yale J Biol Med. 2012;85:19–28.
Stancu C, Sima A. Statins: mechanism of action and effects. J Cell Mol Med. 2001;5:378–87.
Katoh M, Katoh M. Human FOX gene family (Review). Int J Oncol. 2004;25:1495–1500.
Goretsky T, Bradford EM, Ye Q, Lamping OF, Vanagunas T, Moyer MP, et al. Beta-catenin cleavage enhances transcriptional activation. Sci Rep. 2018;8:671.
Lheureux S, Gourley C, Vergote I, Oza AM. Epithelial ovarian cancer. Lancet. 2019;393:1240–53.
Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer Biol Med. 2017;14:9–32.
Zhu F, Xu X, Shi B, Zeng L, Wang L, Wu X, et al. The positive predictive value of low-density lipoprotein for recurrence-free survival in ovarian cancer. Int J Gynaecol Obstet. 2018;143:232–8.
Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–92.
Huang Y, Hong W, Wei X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J Hematol Oncol. 2022;15:129.
Huang T, Song X, Xu D, Tiek D, Goenka A, Wu B, et al. Stem cell programs in cancer initiation, progression, and therapy resistance. Theranostics. 2020;10:8721–43.
Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–20.
Najafi M, Farhood B, Mortezaee K. Cancer stem cells (CSCs) in cancer progression and therapy. J Cell Physiol. 2019;234:8381–95.
Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14:611–29.
Lambert AW, Weinberg RA. Linking EMT programmes to normal and neoplastic epithelial stem cells. Nat Rev Cancer. 2021;21:325–38.
Montecucco F, Quercioli A, Mach F. Ezetimibe/simvastatin. Expert Opin Drug Saf. 2009;8:715–25.
Wang J, Li W, Zhao Y, Kang D, Fu W, Zheng X, et al. Members of FOX family could be drug targets of cancers. Pharm Ther. 2018;181:183–96.
Pei Y, Wang P, Liu H, He F, Ming L. FOXQ1 promotes esophageal cancer proliferation and metastasis by negatively modulating CDH1. Biomed Pharmacother. 2015;74:89–94.
Peng X, Luo Z, Kang Q, Deng D, Wang Q, Peng H, et al. FOXQ1 mediates the crosstalk between TGF-β and Wnt signaling pathways in the progression of colorectal cancer. Cancer Biol Ther. 2015;16:1099–109.
Zhang J, Liu Y, Zhang J, Cui X, Li G, Wang J, et al. FOXQ1 promotes gastric cancer metastasis through upregulation of Snail. Oncol Rep. 2016;35:3607–13.
Sehrawat A, Kim SH, Vogt A, Singh SV. Suppression of FOXQ1 in benzyl isothiocyanate-mediated inhibition of epithelial-mesenchymal transition in human breast cancer cells. Carcinogenesis. 2013;34:864–73.
Wang X, Zhu X. Tumor forkhead box Q1 is elevated, correlates with increased tumor size, international federation of gynecology and obstetrics stage but worse overall survival in epithelial ovarian cancer patients. Cancer Biother Radiopharm. 2022;37:837–42.
Luo Y, Wang J, Wang F, Liu X, Lu J, Yu X, et al. Foxq1 promotes metastasis of nasopharyngeal carcinoma by inducing vasculogenic mimicry via the EGFR signaling pathway. Cell Death Dis. 2021;12:411.
Pizzolato G, Moparthi L, Söderholm S, Cantù C, Koch S. The oncogenic transcription factor FOXQ1 is a differential regulator of Wnt target genes. J Cell Sci 2022;135.
Zhu X, Hua E, Tu Q, Liu M, Xu L, Feng J. Foxq1 promotes alveolar epithelial cell death through Tle1-mediated inhibition of the NF-κB signaling pathway. Am J Respir Cell Mol Biol. 2024;71:53–65.
Sun X, Peng X, Cao Y, Zhou Y, Sun Y. ADNP promotes neural differentiation by modulating Wnt/β-catenin signaling. Nat Commun. 2020;11:2984.
Jin B, Wang C, Li J, Du X, Ding K, Pan J. Anthelmintic niclosamide disrupts the interplay of p65 and FOXM1/β-catenin and eradicates leukemia stem cells in chronic myelogenous leukemia. Clin Cancer Res. 2017;23:789–803.
Katoh M, Katoh M. WNT signaling and cancer stemness. Essays Biochem. 2022;66:319–31.
Bian J, Dannappel M, Wan C, Firestein R. Transcriptional regulation of Wnt/β-catenin pathway in colorectal cancer. Cells. 2020;9.
Nguyen VHL, Hough R, Bernaudo S, Peng C. Wnt/β-catenin signalling in ovarian cancer: insights into its hyperactivation and function in tumorigenesis. J Ovarian Res. 2019;12:122.
Acknowledgements
This work was supported by Zhejiang Provincial Natural Science Foundation of China (Grant number LY24H260002), the National Natural Science Foundation of China (Grant numbers 82303377, 32370584), Zhejiang University School of Public Health Interdisciplinary Research Innovation Team Development Project. We thank for the technical support by the Core Facilities and Qiong Huang, Zhejiang University School of Medicine.
Author information
Authors and Affiliations
Contributions
TS was responsible for methodology, formal analysis, data curation, writing the original draft and visualization. CKL was responsible for data curation and funding acquisition. ZF and FZQ contributed to investigation and formal analysis. NYQ and LXX were responsible for software. NH, YXY, and CZY contributed to validation. LWG, XDJ, and WYH contributed to conceptualization, supervision and funding acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, S., Chen, K., Zheng, F. et al. High serum LDL promotes EMT and stemness through LDLR/FOXQ1/NF-κB1 pathway in epithelial ovarian cancer. Oncogene 44, 4587–4600 (2025). https://doi.org/10.1038/s41388-025-03609-4
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41388-025-03609-4