Abstract
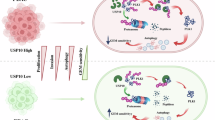
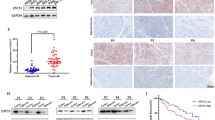
Gemcitabine resistance remains a major obstacle in the treatment of pancreatic adenocarcinoma (PDAC). Through gain- and loss-of-function experiments, we identified USP10 as a positive regulator of tumor growth and gemcitabine resistance. Mechanistically, we demonstrate that USP10 stabilizes IGF2BP3 by removing its K48- and K63-linked ubiquitin chains, thereby inhibiting proteasomal degradation. The stabilized IGF2BP3 binds to and enhances the stability of STEAP3 mRNA in an m⁶A-dependent manner. Upregulation of STEAP3 suppresses ferroptosis by increasing glutathione levels and reducing lipid peroxidation, ultimately promoting tumor proliferation and gemcitabine resistance. Our study identifies the USP10-IGF2BP3-STEAP3 axis as a critical mechanism underlying chemoresistance in pancreatic cancer, suggesting that targeting USP10 may offer a promising therapeutic strategy for overcoming gemcitabine resistance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The datasets generated during or analyzed during the current study are available from the corresponding authors on reasonable request. Figshare :https://doi.org/10.6084/m9.figshare.30017347.
References
Siegel R, Miller K, Wagle N, Jemal A. Cancer statistics, 2023. CA: A Cancer J Clin. 2023;73:17–48.
Qiu H, Cao H, Cao S, Xu R. Cancer incidence, mortality, and burden in China: a time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun (Lond, Engl). 2021;41:1037–48.
Chen X, Zeh X, Zeh H, Kang H, Kang R, Kroemer R, et al. Cell death in pancreatic cancer: from pathogenesis to therapy. Nat Rev Gastroenterol Hepatol. 2021;18:804–23.
Park W, Chawla W, Chawla A, O’Reilly E. Pancreatic cancer: a review. JAMA. 2021;326:851–62.
Tempero M, Malafa M, Al-Hawary M, Behrman S, Benson A, Cardin D, et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw: JNCCN. 2021;19:439–57.
Mini E, Nobili S, Caciagli B, Landini I, Mazzei T. Cellular pharmacology of gemcitabine. Ann Oncol. 2006;17 Suppl 5:v7-12.
Wood L, Canto L, Canto M, Jaffee M, Jaffee E, Simeone D, et al. Pancreatic cancer: pathogenesis, screening, diagnosis, and treatment. Gastroenterology. 2022;163:386–402.e1.
Waks A, Winer E. Breast Cancer Treatment: A Review. JAMA. 2019;321:288–300.
Lenis AT, Lec PM, Chamie K, Mshs MD. Bladder Cancer: A Review. JAMA. 2020;324:1980–91.
Ho W, Jaffee W, Jaffee E, Zheng L. The tumour microenvironment in pancreatic cancer - clinical challenges and opportunities. Nat Rev Clin Oncol. 2020;17:527–40.
Capula M, Per M, Perán M, Xu M, Xu G, Donati G, et al. Role of drug catabolism, modulation of oncogenic signaling and tumor microenvironment in microbe-mediated pancreatic cancer chemoresistance. Drug Resist Updat. 2022;64:100864.
McMillin DW, Negri JM, Mitsiades CS. The role of tumour-stromal interactions in modifying drug response: challenges and opportunities. Nat Rev Drug Discov. 2013;12:217–28.
Wei L, Lin L, Lin Q, Lu Q, Lu Y, Li Y, et al. Cancer-associated fibroblasts-mediated ATF4 expression promotes malignancy and gemcitabine resistance in pancreatic cancer via the TGF-β1/SMAD2/3 pathway and ABCC1 transactivation. Cell Death Dis. 2021;12:334.
Shigeta K, Hasegawa K, Hasegawa M, Hishiki M, Hishiki T, Naito T, et al. IDH2 stabilizes HIF-1α-induced metabolic reprogramming and promotes chemoresistance in urothelial cancer. EMBO J. 2023;42:e110620.
Yao H, Song H, Song W, Cao W, Cao R, Ye R, et al. An EGFR/HER2-targeted conjugate sensitizes gemcitabine-sensitive and resistant pancreatic cancer through different SMAD4-mediated mechanisms. Nat Commun. 2022;13:5506.
Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol cell Biol. 2021;22:266–82.
Wang Y, Wu Y, Wu X, Ren X, Ren Z, Li Z, et al. Overcoming cancer chemotherapy resistance by the induction of ferroptosis. Drug Resist Updat. 2023;66:100916.
Zhang C, Liu C, Liu X, Jin X, Jin S, Chen S, et al. Ferroptosis in cancer therapy: a novel approach to reversing drug resistance. Mol Cancer. 2022;21:47.
Qi R, Bai R, Bai Y, Li Y, Li K, Liu K, et al. Cancer-associated fibroblasts suppress ferroptosis and induce gemcitabine resistance in pancreatic cancer cells by secreting exosome-derived ACSL4-targeting miRNAs. Drug Resist Updat. 2023;68:100960.
Liu J, Zhang S, Cao L, Zhang N, Guo Q, Zou Y, et al. The deubiquitination-PARylation positive feedback loop of the USP10-PARP1 axis promotes DNA damage repair and affects therapeutic efficacy of PARP1 inhibitor. Oncogene. 2025;44:2515–29.
Cao Y, Xie Y, Xie L, Tong L, Tong B, Chu B, et al. Targeting USP10 induces degradation of oncogenic ANLN in esophageal squamous cell carcinoma. Cell Death Differ. 2023;30:527–43.
Li F, He Z, Zhang X, Gao D, Xu R, Zhang Z, et al. USP10 promotes cell proliferation, migration, and invasion in NSCLC through deubiquitination and stabilization of EIF4G1. Sci Rep. 2024;14:23685.
Yuan T, Chen Z, Yan F, Qian M, Luo H, Ye S, et al. Deubiquitinating enzyme USP10 promotes hepatocellular carcinoma metastasis through deubiquitinating and stabilizing Smad4 protein. Mol Oncol. 2020;14:197–210.
Zhai S, Lin S, Lin J, Ji J, Ji Y, Zhang Y, et al. A microprotein N1DARP encoded by LINC00261 promotes Notch1 intracellular domain (N1ICD) degradation via disrupting USP10-N1ICD interaction to inhibit chemoresistance in Notch1-hyperactivated pancreatic cancer. Cell Discov. 2023;9:95.
Liu X, Zhang S, An Y, Xu B, Yan G, Sun M. USP10/XAB2/ANXA2 axis promotes DNA damage repair to enhance chemoresistance to oxaliplatin in colorectal cancer. J Exp Clin Cancer Res. 2025;44:94.
Luo P, Qin P, Qin C, Zhu C, Zhu L, Fang L, et al. Ubiquitin-specific peptidase 10 (USP10) inhibits hepatic steatosis, insulin resistance, and inflammation through Sirt6. Hepatol (Balt, Md). 2018;68:1786–803.
Harrigan JA, Jacq X, Martin NM, Jackson SP. Deubiquitylating enzymes and drug discovery: emerging opportunities. Nat Rev Drug Discov. 2018;17:57–78.
Hu C, Zhang M, Moses N, Hu CL, Polin L, Chen W, et al. The USP10-HDAC6 axis confers cisplatin resistance in non-small cell lung cancer lacking wild-type p53. Cell Death Dis. 2020;11:328.
Sun L, Yu J, Guinney J, Qin B, Sinicrope FA. USP10 regulates ZEB1 ubiquitination and protein stability to inhibit ZEB1-mediated colorectal cancer metastasis. Mol Cancer Res. 2023;21:578–90.
Zhao X, Ma Y, Li J, Sun X, Sun Y, Qu F, et al. The AEG-1-USP10-PARP1 axis confers radioresistance in esophageal squamous cell carcinoma via facilitating homologous recombination-dependent DNA damage repair. Cancer Lett. 2023;577:216440.
Zhu H, Yan F, Yuan T, Qian M, Zhou T, Dai X, et al. USP10 promotes proliferation of hepatocellular carcinoma by deubiquitinating and stabilizing YAP/TAZ. Cancer Res. 2020;80:2204–16.
Wang X, Xia S, Li H, Wang X, Li C, Chao Y, et al. The deubiquitinase USP10 regulates KLF4 stability and suppresses lung tumorigenesis. Cell Death Differ. 2020;27:1747–64.
Yuan J, Luo K, Zhang L, Cheville J, Lou Z. USP10 regulates p53 localization and stability by deubiquitinating p53. Cell. 2010;140:384–96.
Dikic I, Schulman BA. An expanded lexicon for the ubiquitin code. Nat Rev Mol Cell Biol. 2023;24:273–87.
Cockram PE, Kist M, Prakash S, Chen SH, Wertz IE, Vucic D. Ubiquitination in the regulation of inflammatory cell death and cancer. Cell Death Differ. 2021;28:591–605.
Li K, Guo J, Ming Y, Chen S, Zhang T, Ma H, et al. A circular RNA activated by TGFβ promotes tumor metastasis through enhancing IGF2BP3-mediated PDPN mRNA stability. Nat Commun. 2023;14:6876.
Chen B, Huang R, Xia T, Wang C, Xiao X, Lu S, et al. The m⁶A reader IGF2BP3 preserves NOTCH3 mRNA stability to sustain Notch3 signaling and promote tumor metastasis in nasopharyngeal carcinoma. Oncogene. 2023;42:3564–74.
Ge L, Rui Y, Wang C, Wu Y, Wang H, Wang J. The RNA m⁶A reader IGF2BP3 regulates NFAT1/IRF1 axis-mediated anti-tumor activity in gastric cancer. Cell Death Dis. 2024;15:192.
Zhang X, Shi L, Sun HD, Wang ZW, Xu F, Wei JF, et al. IGF2BP3 mediates the mRNA degradation of NF1 to promote triple-negative breast cancer progression via an m⁶A-dependent manner. Clin Transl Med. 2023;13:e1427.
Xu X, Zhang Y, Wu S, Wu Y, Lin X, Chen K, et al. Hepatitis B virus promotes angiogenesis in hepatocellular carcinoma by increasing m⁶A modification of VEGFA mRNA via IGF2BP3. J Med Virol. 2025;97:e70356.
Zhao W, Lu D, Liu L, Cai J, Zhou Y, Yang Y, et al. Insulin-like growth factor 2 mRNA binding protein 3 (IGF2BP3) promotes lung tumorigenesis via attenuating p53 stability. Oncotarget. 2017;8:93672–87.
Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31:107–25.
Lomphithak T, Sae-Fung A, Sprio S, Tampieri A, Jitkaew S, Fadeel B. Exploiting the ferroaddiction of pancreatic cancer cells using Fe-doped nanoparticles. Nanomedicine. 2024;55:102714.
Deng P, Li J, Lu Y, Hao R, He M, Li M, et al. Chronic cadmium exposure triggered ferroptosis by perturbing the STEAP3-mediated glutathione redox balance linked to altered metabolomic signatures in humans. Sci Total Environ. 2023;905:167039.
Lan J, Zhang W, Zeng K, Li C, He J, Li X, et al. β-elemene promotes ferroptosis to improve the sensitivity of imatinib in gastrointestinal stromal tumours by targeting N6AMT1. Clin Transl Med. 2025;15:e70438.
Cañeque T, Baron L, Müller S, Carmona A, Colombeau L, Versini A, et al. Activation of lysosomal iron triggers ferroptosis in cancer. Nature. 2025;642:492–500.
Wang Y, Wang Y, Patel H, Chen J, Wang J, Chen ZS, et al. Epigenetic modification of m⁶A regulator proteins in cancer. Mol Cancer. 2023;22:102.
Pan Y, Gu Y, Liu T, Zhang Q, Yang F, Duan L, et al. Epitranscriptic regulation of HRAS by N6-methyladenosine drives tumor progression. Proc Natl Acad Sci USA. 2023;120:e2302291120.
Zheng M, Wang S, Tang K, Kong R, Wang X, Zhou J, et al. The CYLD-PARP1 feedback loop regulates DNA damage repair and chemosensitivity in breast cancer cells. Proc Natl Acad Sci USA. 2025;122:e2413890121.
Zhang Q, Zhang ZY, Du H, Li SZ, Tu R, Jia YF, et al. DUB3 deubiquitinates and stabilizes NRF2 in chemotherapy resistance of colorectal cancer. Cell Death Differ. 2019;26:2300–13.
Du L, Li Y, Kang M, Feng M, Ren Y, Dai H, et al. USP48 is upregulated by Mettl14 to attenuate hepatocellular carcinoma via regulating SIRT6 stabilization. Cancer Res. 2021;81:3822–34.
Xu MH, Zheng YM, Liang BG, Xu WX, Cao J, Wang P, et al. Deubiquitination of CIB1 by USP14 promotes lenvatinib resistance via the PAK1-ERK1/2 axis in hepatocellular carcinoma. Int J Biol Sci. 2024;20:3269–84.
Cervia LD, Shibue T, Borah AA, Gaeta B, He L, Leung L, et al. A ubiquitination cascade regulating the integrated stress response and survival in carcinomas. Cancer Discov. 2023;13:766–95.
Du X, Yu R, Yan C, Dong P, Wei C, Wang B, et al. USP10 promotes the progression and attenuates gemcitabine chemotherapy sensitivity via stabilizing PLK1 in PDAC. Cell Death Dis. 2025;16:449.
Acknowledgements
We thank the Institute of Gastrointestinal Disease, the Sixth Affiliated Hospital of Sun Yat-Sen University for support with equipment and site.
Funding
This study was supported by National Natural Science Youth Foundation of China (Z201901022021121076), Discipline construction fund of the Sixth Affiliated Hospital of Sun Yat-Sen University (X202102172026091185), National Natural Science Youth Foundation of China (No. Z201901022021121076; No. 82403535) and China postdoctoral science foundation (2023M744076, 2024T171079).
Author information
Authors and Affiliations
Contributions
Y-lL, C-RZ, and J-YW performed most of the cellular, biochemical, and animal experiments. Z-JL and Z-L partially designed the experimental plans and contributed to the cellular and biochemical experiments. T-jY, Z-PC, H-LJ, and J-DY partially contributed to the data analysis and provided the technological support for animal experiments. G-LL, Y-LW, and Z-YL designed experiments, supervised the project analyzed results, and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was conducted in compliance with international, national, and institutional regulations regarding animal experiments, clinical studies, and biodiversity rights. All participants signed an informed consent form and this information was submitted to the ethics committee for the assignment of an ethical approval code. The Institutional Review Board of The Sixth Affiliated Hospital of Sun Yat-Sen University approved this study. Approval number: IACUC-2022081101. The study was performed in accordance with the Declaration of Helsinki.
Consent for publication
All authors reviewed and approved the manuscript prior to submission.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, YL., Zhong, CR., Wu, JY. et al. USP10 promotes cell proliferation and gemcitabine resistance in pancreatic cancer by the regulation of IGF2BP3-STEAP3. Oncogene 45, 383–397 (2026). https://doi.org/10.1038/s41388-025-03654-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41388-025-03654-z