Abstract
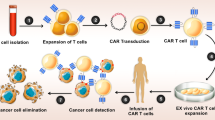
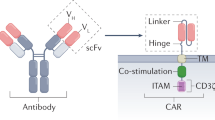
Ovarian cancer (OC) remains a lethal malignancy with limited treatment options owing to antigen heterogeneity and an immunosuppressive tumor microenvironment (TME). Here, we designed a unique chimeric Antigen Receptor T-Cell (CAR-T) construct (B4M3) that integrates an anti-MSLN scFv linked to the CD3ζ activation domain and an anti-B7H3 scFv linked to the 4-1BB co-stimulatory domain. In vitro, B4M3 CAR-T cells exhibited robust cytotoxicity against OC cell lines with enhanced degranulation (CD107a) and efficient tumor cell killing, even at low effector-to-target ratios. In vivo, B4M3 CAR-T cells significantly inhibited tumor growth and prolonged survival and demonstrated superior tumor infiltration and persistence in OC xenograft models. Imaging mass cytometry (IMC) revealed that B4M3 treatment reshaped the TME, increased cytotoxic T lymphocyte (CTL) infiltration, and reduced regulatory T cells (Tregs). Mechanistically, B4M3 therapy upregulated TGF-β, promoting Th17 differentiation and CTL recruitment, thereby enhancing anti-tumor immunity. Our findings demonstrate that B4M3 CAR-T cells effectively address antigen heterogeneity and enhance therapeutic efficacy in OC, thereby offering a promising strategy for solid tumor immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The authors declare that all data supporting the results of this study are available in the paper and its supplementary information. Source data for the figures in this study are available from the corresponding author upon request.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68:284–96.
Bouchkouj N, Kasamon YL, de Claro RA, George B, Lin X, Lee S, et al. FDA approval summary: axicabtagene ciloleucel for relapsed or refractory large b-cell lymphoma. Clin Cancer Res. 2019;25:1702–8.
Munshi NC, Anderson LDJ, Shah N, Madduri D, Berdeja J, Lonial S, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384:705–16.
Wagner J, Wickman E, DeRenzo C, Gottschalk S. CAR t cell therapy for solid tumors: bright future or dark reality?. Mol Ther. 2020;28:2320–39.
Marofi F, Motavalli R, Safonov VA, Thangavelu L, Yumashev AV, Alexander M, et al. CAR t cells in solid tumors: challenges and opportunities. Stem Cell Res Ther. 2021;12:81.
Klampatsa A, Dimou V, Albelda SM. Mesothelin-targeted CAR-t cell therapy for solid tumors. Expert Opin Biol Ther. 2021;21:473–86.
Scales SJ, Gupta N, Pacheco G, Firestein R, French DM, Koeppen H, et al. An antimesothelin-monomethyl auristatin e conjugate with potent antitumor activity in ovarian, pancreatic, and mesothelioma models. Mol Cancer Ther. 2014;13:2630–40.
Winter JM, Tang LH, Klimstra DS, Brennan MF, Brody JR, Rocha FG, et al. A novel survival-based tissue microarray of pancreatic cancer validates MUC1 and mesothelin as biomarkers. PLoS ONE. 2012;7:e40157.
Ho M, Bera TK, Willingham MC, Onda M, Hassan R, FitzGerald D, et al. Mesothelin expression in human lung cancer. Clin Cancer Res. 2007;13:1571–5.
Baba K, Ishigami S, Arigami T, Uenosono Y, Okumura H, Matsumoto M, et al. Mesothelin expression correlates with prolonged patient survival in gastric cancer. J Surg Oncol. 2012;105:195–9.
Obulhasim G, Fujii H, Matsumoto T, Yasen M, Abe M, Matsuoka S, et al. Mesothelin gene expression and promoter methylation/hypomethylation in gynecological tumors. Eur J Gynaecol Oncol. 2010;31:63–71.
Tozbikian G, Brogi E, Kadota K, Catalano J, Akram M, Patil S, et al. Mesothelin expression in triple negative breast carcinomas correlates significantly with basal-like phenotype, distant metastases and decreased survival. PLoS One. 2014;9:e114900.
Zhao B, Li H, Xia Y, Wang Y, Wang Y, Shi Y, et al. Immune checkpoint of b7-h3 in cancer: from immunology to clinical immunotherapy. J Hematol Oncol. 2022;15:153.
Majzner RG, Theruvath JL, Nellan A, Heitzeneder S, Cui Y, Mount CW, et al. CAR t cells targeting b7-h3, a pan-cancer antigen, demonstrate potent preclinical activity against pediatric solid tumors and brain tumors. Clin Cancer Res. 2019;25:2560–74.
Beatty GL, O’Hara MH, Lacey SF, Torigian DA, Nazimuddin F, Chen F, et al. Activity of mesothelin-specific chimeric antigen receptor t cells against pancreatic carcinoma metastases in a phase 1 trial. Gastroenterology. 2018;155:29–32.
Vitanza NA, Wilson AL, Huang W, Seidel K, Brown C, Gustafson JA, et al. Intraventricular b7-h3 CAR t cells for diffuse intrinsic pontine glioma: preliminary first-in-human bioactivity and safety. Cancer Discov. 2023;13:114–31.
Saif WM, Cassier PA, Curigliano G, Daniele G, Hilton JF, Koganemaru S, et al. A phase 1 dose escalation/expansion study of GSK5764227 (GSK’227), a b7-homolog 3 (b7-h3) protein targeted antibody-drug conjugate (ADC), in patients with advanced solid tumors, including gastrointestinal (GI) cancers. J Clin Oncol. 2025;43:TPS84.
Zhang E, Yang P, Gu J, Wu H, Chi X, Liu C, et al. Recombination of a dual-CAR-modified t lymphocyte to accurately eliminate pancreatic malignancy. J Hematol Oncol. 2018;11:102.
Yin Y, Rodriguez JL, Li N, Thokala R, Nasrallah MP, Hu L, et al. Locally secreted BiTEs complement CAR t cells by enhancing killing of antigen heterogeneous solid tumors. Mol Ther. 2022;30:2537–53.
Hegde M, Mukherjee M, Grada Z, Pignata A, Landi D, Navai SA, et al. Tandem CAR t cells targeting HER2 and IL13ralpha2 mitigate tumor antigen escape. J Clin Invest. 2016;126:3036–52.
Choi BD, Yu X, Castano AP, Bouffard AA, Schmidts A, Larson RC, et al. CAR-t cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat Biotechnol. 2019;37:1049–58.
Lanitis E, Poussin M, Klattenhoff AW, Song D, Sandaltzopoulos R, June CH, et al. Chimeric antigen receptor t cells with dissociated signaling domains exhibit focused antitumor activity with reduced potential for toxicity in vivo. Cancer Immunol Res. 2013;1:43–53.
Ji F, Yu J, Zhu Y, Lin H, Gao K, Rao M, et al. B4m3 CAR-t cell enhance antitumor activity and non-tumor toxicity in ovarian cancer. Int Immunopharmacol. 2025;159:114919.
Ji F, Xu L, Long K, Zhang F, Zhang M, Lu X, et al. Rabies virus glycoprotein 29 (RVG29) promotes CAR-t immunotherapy for glioma. Transl Res. 2023;259:1–12.
Park AK, Fong Y, Kim S, Yang J, Murad JP, Lu J, et al. Effective combination immunotherapy using oncolytic viruses to deliver CAR targets to solid tumors. Sci Transl Med. 2020;12:eaaz1863.
Zhai Y, Du Y, Li G, Yu M, Hu H, Pan C, et al. Trogocytosis of CAR molecule regulates CAR-t cell dysfunction and tumor antigen escape. Signal Transduct Target Ther. 2023;8:457.
Rodriguez-Marquez P, Calleja-Cervantes ME, Serrano G, Oliver-Caldes A, Palacios-Berraquero ML, Martin-Mallo A, et al. CAR density influences antitumoral efficacy of BCMA CAR t cells and correlates with clinical outcome. Sci Adv. 2022;8:eabo0514.
Ruella M, Korell F, Porazzi P, Maus MV. Mechanisms of resistance to chimeric antigen receptor-t cells in haematological malignancies. Nat Rev Drug Discov. 2023;22:976–95.
Hu Y, Hou J, Jiang Z, Lin Q. Mechanisms of resistance to CAR-t cell therapy in multiple myeloma: latest updates from the 2024 ASH annual meeting. Exp Hematol Oncol. 2025;14:45.
Marchal I. FOXO1 enhances CAR t cell fitness and function. Nat Biotechnol. 2024;42:699.
Stewart C, Ralyea C, Lockwood S. Ovarian cancer: an integrated review. Semin Oncol Nurs. 2019;35:151–6.
Bu L, Yan J, Wang Z, Ruan H, Chen Q, Gunadhi V, et al. Advances in drug delivery for post-surgical cancer treatment. Biomaterials. 2019;219:119182.
Sommer C, Boldajipour B, Kuo TC, Bentley T, Sutton J, Chen A, et al. Preclinical evaluation of allogeneic CAR t cells targeting BCMA for the treatment of multiple myeloma. Mol Ther. 2019;27:1126–38.
Flugel CL, Majzner RG, Krenciute G, Dotti G, Riddell SR, Wagner DL, et al. Overcoming on-target, off-tumour toxicity of CAR t cell therapy for solid tumours. Nat Rev Clin Oncol. 2023;20:49–62.
Wang Y, Buck A, Piel B, Zerefa L, Murugan N, Coherd CD, et al. Affinity fine-tuning anti-CAIX CAR-t cells mitigate on-target off-tumor side effects. Mol Cancer. 2024;23:56.
Majzner RG, Rietberg SP, Sotillo E, Dong R, Vachharajani VT, Labanieh L, et al. Tuning the antigen density requirement for CAR t-cell activity. Cancer Discov. 2020;10:702–23.
Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, et al. CD22-targeted CAR t cells induce remission in b-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24:20–8.
Shi X, Yang J, Deng S, Xu H, Wu D, Zeng Q, et al. TGF-beta signaling in the tumor metabolic microenvironment and targeted therapies. J Hematol Oncol. 2022;15:135.
Batlle E, Massague J. Transforming growth factor-beta signaling in immunity and cancer. Immunity. 2019;50:924–40.
Lan Y, Moustafa M, Knoll M, Xu C, Furkel J, Lazorchak A, et al. Simultaneous targeting of TGF-beta/PD-l1 synergizes with radiotherapy by reprogramming the tumor microenvironment to overcome immune evasion. Cancer Cell. 2021;39:1388–403.
Doan AE, Mueller KP, Chen AY, Rouin GT, Chen Y, Daniel B, et al. FOXO1 is a master regulator of memory programming in CAR t cells. Nature. 2024;629:211–8.
Carlson CM, Endrizzi BT, Wu J, Ding X, Weinreich MA, Walsh ER, et al. Kruppel-like factor 2 regulates thymocyte and t-cell migration. Nature. 2006;442:299–302.
Jung YW, Kim HG, Perry CJ, Kaech SM. CCR7 expression alters memory CD8 t-cell homeostasis by regulating occupancy in IL-7- and IL-15-dependent niches. Proc Natl Acad Sci USA. 2016;113:8278–83.
Dominguez CX, Muller S, Keerthivasan S, Koeppen H, Hung J, Gierke S, et al. Single-cell RNA sequencing reveals stromal evolution into LRRC15(+) myofibroblasts as a determinant of patient response to cancer immunotherapy. Cancer Discov. 2020;10:232–53.
Levstek L, Janzic L, Ihan A, Kopitar AN. Biomarkers for prediction of CAR t therapy outcomes: current and future perspectives. Front Immunol. 2024;15:1378944.
Garcia-Murillas I, Schiavon G, Weigelt B, Ng C, Hrebien S, Cutts RJ, et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci Transl Med. 2015;7:302ra133.
Li S, Lai H, Liu J, Liu Y, Jin L, Li Y, et al. Circulating tumor DNA predicts the response and prognosis in patients with early breast cancer receiving neoadjuvant chemotherapy. JCO Precis Oncol. 2020;4:PO.19.00292.
Hope JL, Stairiker CJ, Bae E, Otero DC, Bradley LM. Striking a balance-cellular and molecular drivers of memory t cell development and responses to chronic stimulation. Front Immunol. 2019;10:1595.
Michelini Hess, Doedens R, Goldrath AL, Hedrick AW. SM. Differentiation of CD8 memory t cells depends on foxo1. J Exp Med. 2013;210:1189–200.
Zhang H, Yang Z, Yuan W, Liu J, Luo X, Zhang Q, et al. Sustained AhR activity programs memory fate of early effector CD8(+) t cells. Proc Natl Acad Sci USA. 2024;121:e2317658121.
Agliardi G, Dias J, Rampotas A, Garcia J, Roddie C. Accelerating and optimising CAR t-cell manufacture to deliver better patient products. Lancet Haematol. 2025;12:e57–67.
Rupp LJ, Schumann K, Roybal KT, Gate RE, Ye CJ, Lim WA, et al. CRISPR/cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor t cells. Sci Rep. 2017;7:737.
Acknowledgements
We are grateful to the staff in Biobank of Zhongda Hospital Affiliated to Southeast University for technical assistance. The authors would like to thank Shengmaiyi (Nanjing) Biotechnology Co. for providing infrastructure and financial support.
Author contributors
FJ, YS: Conception and design; FJ, KY, BD, YZ: Acquisition of data; FJ, MR, KG, HL, SL: Analysis and interpretation of data; FJ, KG, YS, KY: Writing, review, and revision of the manuscript; FJ, BD, YS: Administrative, technical, or material support; ZX, YS: Study supervision. All authors have read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (82303764, 82372126, and 82072078), the China Postdoctoral Science Foundation (2023M740616, 2025T180583, and 2024M750460), Young Elite Scientists Sponsorship Program by Jiangsu Association for Science and Technology (JSTJ-2025-502), the Beijing Xisike Clinical Oncology Research Foundation (Y-Young2024-0102, Y-zai2022/ms-0126), Noncommunicable Chronic Diseases-National Science and Technology Major Project (2025ZD0545600), the Research Talent Cultivation Program of Zhongda Hospital Affiliated to Southeast University (CZXM-GSP-RC06), and Jiangsu Province High-Level Hospital Pairing Assistance Construction Funds from Zhongda Hospital Affiliated to Southeast University (zdyyxy35).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All methods were performed in accordance with the relevant guidelines and regulations. All animal experiments were approved by the Institutional Animal Care and Use Committee of Southeast University (approval number 20230313001), in accordance with institutional guidelines. No human subjects were involved in this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ji, F., Yan, K., Ding, B. et al. Dual-target CAR-T therapy for ovarian cancer: synergistic targeting of MSLN and B7H3 enhances anti-tumor efficacy and overcomes antigen heterogeneity. Oncogene 45, 446–458 (2026). https://doi.org/10.1038/s41388-025-03663-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41388-025-03663-y