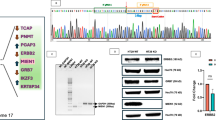
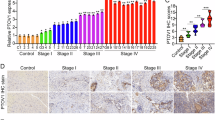
Abstract
Metastasis to distant organs represents the most fatal prognostic factor for colorectal cancer (CRC). The distant metastasis of tumor cells results from the collaborative effort of multiple subcellular structures, with dynamic cytoskeletal remodeling underlying this entire process. Here, we found that knockdown of KRT81 expression (shKRT81) inhibited the proliferation, invasion, and migration, while ectopic overexpression of KRT81 enhanced the CRC cells migration. Furthermore, we identified a potential downstream effector of KRT81, ezrin, a member of the ezrin/radixin/moesin (ERM) protein family that regulates cell morphology and motility. Phenotypically, the shKRT81 attenuated ezrin protein expression and reduced the number and length of filopodia in CRC cells, which were restored when KRT81 was re-overexpressed. Mechanistically, KRT81 formed a complex with ezrin, and recruitment of ezrin to the membrane and phosphorylation at the Thr567 residue were significantly abolished in shKRT81 cells. Interestingly, we found that Myosin 1B (MYO1B) might provide the driving force for the recruitment of ezrin. Notably, combinatorial inhibition (shKRT81 + ezrin-specific inhibitor) exerted significantly greater suppression of CRC cell migration and invasion than either intervention alone. Consistently, KRT81 expression was increased in CRC, and relatively high expression of KRT81 was associated with a poor prognosis. In summary, we identified a novel regulatory axis that involves KRT81, MYO1B, and ezrin, which regulates filopodia formation and migration behavior in CRC. Therefore, KRT81 may serve as a therapeutic target for CRC.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All data associated with the current study are presented in the manuscript or Supplementary Data. Original materials are available from the corresponding author on rational request.
References
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–63.
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467–80.
Cervantes A, Adam R, Rosello S, Arnold D, Normanno N, Taieb J, et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:10–32.
Xie YH, Chen YX, Fang JY. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Tar. 2020;5:22.
Biller LH, Schrag D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA. 2021;325:669–85.
Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400.
Bose D, Meric-Bernstam F, Hofstetter W, Reardon DA, Flaherty KT, Ellis LM. Vascular endothelial growth factor targeted therapy in the perioperative setting: implications for patient care. Lancet Oncol. 2010;11:373–82.
Tabernero J, Grothey A, Van Cutsem E, Yaeger R, Wasan H, Yoshino T, et al. Encorafenib Plus Cetuximab as a New Standard of Care for Previously Treated BRAF V600E–Mutant Metastatic Colorectal Cancer: Updated Survival Results and Subgroup Analyses from the BEACON Study. J Clin Oncol. 2021;39:273–84.
Diaz LA, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2022;23:659–70.
Gerstberger S, Jiang Q, Ganesh K. Metastasis. Cell. 2023;186:1564–79.
Zhan Q, Liu B, Situ X, Luo Y, Fu T, Wang Y, et al. New insights into the correlations between circulating tumor cells and target organ metastasis. Signal Transduct Tar. 2023;8:465.
Liu W, Chen S, Xie W, Wang Q, Luo Q, Huang M, et al. MCCC2 is a novel mediator between mitochondria and telomere and functions as an oncogene in colorectal cancer. Cell Mol Biol Lett. 2023;28:80.
Herrmann H, Bar H, Kreplak L, Strelkov SV, Aebi U. Intermediate filaments: from cell architecture to nanomechanics. Nat Rev Mol Cell Biol. 2007;8:562–73.
Chu PG, Weiss LM. Keratin expression in human tissues and neoplasms. Histopathology. 2002;40:403–39.
Kang DS, Moriarty A, Wang YJ, Thomas A, Hao J, Unger BA, et al. Ectopic Expression of a Truncated Isoform of Hair Keratin 81 in Breast Cancer Alters Biophysical Characteristics to Promote Metastatic Propensity. Adv Sci (Weinh). 2024;11:e2300509.
Schweizer J, Bowden PE, Coulombe PA, Langbein L, Lane EB, Magin TM, et al. New consensus nomenclature for mammalian keratins. J Cell Biol. 2006;174:169–74.
Karantza V. Keratins in health and cancer: more than mere epithelial cell markers. Oncogene. 2011;30:127–38.
Aebi U, Fowler WE, Rew P, Sun TT. The fibrillar substructure of keratin filaments unraveled. J Cell Biol. 1983;97:1131–43.
Herrmann H, Aebi U. Intermediate Filaments: Structure and Assembly. Cold Spring Harb Perspect Biol. 2016;8:a018242.
Werner S, Keller L, Pantel K. Epithelial keratins: Biology and implications as diagnostic markers for liquid biopsies. Mol Asp Med. 2020;72:100817.
Trask DK, Band V, Zajchowski DA, Yaswen P, Suh T, Sager R. Keratins as markers that distinguish normal and tumor-derived mammary epithelial cells. Proc Natl Acad Sci USA. 1990;87:2319–23.
Huang WC, Jang TH, Tung SL, Yen TC, Chan SH, Wang LH. A novel miR-365-3p/EHF/keratin 16 axis promotes oral squamous cell carcinoma metastasis, cancer stemness and drug resistance via enhancing beta5-integrin/c-met signaling pathway. J Exp Clin Cancer Res. 2019;38:89.
Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–24.
Le J, Fu Y, Han Q, Ma Y, Ji H, Wei X, et al. Transcriptome Analysis of the Inhibitory Effect of Sennoside A on the Metastasis of Hepatocellular Carcinoma Cells. Front Pharm. 2020;11:566099.
Győrffy B. Integrated analysis of public datasets for the discovery and validation of survival-associated genes in solid tumors. Innovation. 2024;5:100625.
Barik GK, Sahay O, Paul D, Santra MK. Ezrin gone rogue in cancer progression and metastasis: An enticing therapeutic target. Biochimica Et Biophysica Acta-Rev Cancer. 2022;1877:188753.
Fehon RG, McClatchey AI, Bretscher A. Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol. 2010;11:276–87.
Tran Quang C, Gautreau A, Arpin M, Treisman R. Ezrin function is required for ROCK-mediated fibroblast transformation by the Net and Dbl oncogenes. EMBO J. 2000;19:4565–76.
Xie L, Huang H, Zheng Z, Yang Q, Wang S, Chen Y, et al. MYO1B enhances colorectal cancer metastasis by promoting the F-actin rearrangement and focal adhesion assembly via RhoA/ROCK/FAK signaling. Ann Transl Med. 2021;9:1543.
Gautreau A, Louvard D, Arpin M. Morphogenic effects of ezrin require a phosphorylation-induced transition from oligomers to monomers at the plasma membrane. J Cell Biol. 2000;150:193–203.
Pelaseyed T, Viswanatha R, Sauvanet C, Filter JJ, Goldberg ML, Bretscher A. Ezrin activation by LOK phosphorylation involves a PIP(2)-dependent wedge mechanism. Elife. 2017;6:e22759.
Zaman R, Lombardo A, Sauvanet C, Viswanatha R, Awad V, Bonomo LE, et al. Effector-mediated ERM activation locally inhibits RhoA activity to shape the apical cell domain. J Cell Biol. 2021;220:e202007146.
Qiao J, Tan Y, Liu H, Yang B, Zhang Q, Liu Q, et al. Histone H3K18 and Ezrin Lactylation Promote Renal Dysfunction in Sepsis-Associated Acute Kidney Injury. Adv Sci (Weinh). 2024;11:e2307216.
Yu Y, Khan J, Khanna C, Helman L, Meltzer PS, Merlino G. Expression profiling identifies the cytoskeletal organizer ezrin and the developmental homeoprotein Six-1 as key metastatic regulators. Nat Med. 2004;10:175–81.
Gilbert S, Loranger A, Lavoie JN, Marceau N. Cytoskeleton keratin regulation of FasR signaling through modulation of actin/ezrin interplay at lipid rafts in hepatocytes. Apoptosis. 2012;17:880–94.
Wald FA, Oriolo AS, Casanova ML, Salas PJ. Intermediate filaments interact with dormant ezrin in intestinal epithelial cells. Mol Biol Cell. 2005;16:4096–107.
Gao S, Li E, Cui L, Lu X, Meng L, Yuan H, et al. Sp1 and AP-1 Regulate Expression of the Human Gene VIL2 in Esophageal Carcinoma Cells. J Biol Chem. 2009;284:7995–8004.
Brown L, Waseem A, Cruz IN, Szary J, Gunic E, Mannan T, et al. Desmoglein 3 promotes cancer cell migration and invasion by regulating activator protein 1 and protein kinase C-dependent-Ezrin activation. Oncogene. 2013;33:2363–74.
Zhang RR, Feng XT, Zhan MS, Huang C, Chen K, Tang XY, et al. Transcription Factor Sp1 Promotes the Expression of Porcine Gene. Int J Mol Sci. 2016;17:112.
Wang N, Yang Y, Pang M, Du C, Chen Y, Li S, et al. MicroRNA-135a-5p Promotes the Functional Recovery of Spinal Cord Injury by Targeting SP1 and ROCK. Mol Ther Nucleic Acids. 2020;22:1063–77.
Kang S, Kim J, Park A, Koh M, Shin W, Park G, et al. TRIM40 is a pathogenic driver of inflammatory bowel disease subverting intestinal barrier integrity. Nat Commun. 2023;14:700.
Lin J, Zhang X, Ge W, Duan Y, Zhang X, Zhang Y, et al. Rnd3 Ameliorates Diabetic Cardiac Microvascular Injury via Facilitating Trim40-Mediated Rock1 Ubiquitination. Diabetes. 2025;74:569–84.
Almeida CG, Yamada A, Tenza D, Louvard D, Raposo G, Coudrier E. Myosin 1b promotes the formation of post-Golgi carriers by regulating actin assembly and membrane remodelling at the trans-Golgi network. Nat Cell Biol. 2011;13:779–89.
Coudrier E, Almeida CG. Myosin 1 controls membrane shape by coupling F-Actin to membrane. Bioarchitecture. 2014;1:230–5.
Liu X, Salokas K, Tamene F, Jiu Y, Weldatsadik RG, Öhman T, et al. An AP-MS- and BioID-compatible MAC-tag enables comprehensive mapping of protein interactions and subcellular localizations. Nat Commun. 2018;9:1188.
Go CD, Knight JDR, Rajasekharan A, Rathod B, Hesketh GG, Abe KT, et al. A proximity-dependent biotinylation map of a human cell. Nature. 2021;595:120–4.
Dydensborg AB, Rose AAN, Wilson BJ, Grote D, Paquet M, Giguère V, et al. GATA3 inhibits breast cancer growth and pulmonary breast cancer metastasis. Oncogene. 2009;28:2634–42.
Acknowledgements
We thank the support of the National Key Clinical Discipline in general.
Author information
Authors and Affiliations
Contributions
DC, MH, WX, WL, and MZ provided the study concept and design. DC and MZ supervised this study. MH, WX, WL, and MW performed the experiments. MH, MW, ZO, and WL conducted data analysis, and MH visualized the experimental data. SJ and MH performed the mass spectrometry and data analysis. MH, WX, and WL conducted clinical data and sample collection. DC provided financial support. MH drafted the manuscript. DC, MH, WX, WL, MW, and CL reviewed and prepared the final manuscript. All authors edited and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
This work was financially supported by the Natural Science Foundation of China (Grant No. 31970703), Natural Science Foundation of Guangdong Province (Grant No. 2025A1515010593 and 2022A1515012472). The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics approval and consent to participate
All methods were performed in accordance with the relevant guidelines and regulations. The animal experiments were approved by the Laboratory Animal Center of SYSU-SAH (approval no. IACUC-2023122702), and tumor sizes were within the requirements of the animal center (<2 cm). Our study involving human specimens was approved by the Institutional Ethics Committee of SYSU-SAH (approval no. 2022ZSLYEC-469). All specimens in the SYSU-SAH biobank were collected and deposited after obtaining written informed consent from participants. This was a retrospective analysis of anonymized data, as the authors had no access to identifiable personal information during the analysis. Therefore, the requirement for informed consent was waived to this study by the ethics committee in accordance with applicable guidelines and regulations.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, M., Xie, W., Wu, M. et al. KRT81 promotes metastasis of colorectal cancer by acting as a protein scaffold for ezrin. Oncogene 45, 459–475 (2026). https://doi.org/10.1038/s41388-025-03665-w
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41388-025-03665-w