Abstract
Background
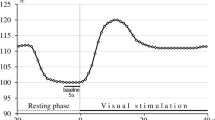
Hypercapnia causes cerebral vasodilation and increased cerebral blood flow (CBF). During prolonged hypercapnia it is unknown whether cerebral vasodilation persists and whether cerebrovascular function is preserved. We investigated the effects of prolonged severe hypercapnia on pial arteriolar diameters (PAD) and cerebrovascular reactivity to vasodilators and vasoconstrictors.
Methods
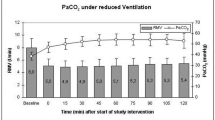
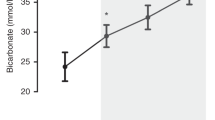
Piglets were anesthetized, intubated and ventilated. Closed cranial windows were implanted to measure PAD. Changes in PAD were documented during hypercapnia (PaCO2 75–80 mm Hg). Cerebrovascular reactivity was documented during normocapnia and at 30, 60, and 120 min of hypercapnia.
Results
Cerebral vasodilation to hypercapnia was sustained over 120 min. Cerebrovascular responses to vasodilators and vasoconstrictors were preserved during hypercapnia. During hypercapnia, vasodilatory responses to second vasodilators were similar to normocapnia, while exposure to vasoconstrictors caused significant vasoconstriction.
Conclusions
Prolonged severe hypercapnia causes sustained vasodilation of pial arteriolar diameters indicative of hyperperfusion. During hypercapnia, cerebral vascular responses to vasodilators and vasoconstrictors were preserved, suggesting that cerebral vascular function remained intact. Of note, cerebral vessels during hypercapnia were capable of further dilation when exposed to additional cerebral vasodilators and, significant vasoconstriction when exposed to vasoconstrictors. Extrapolating these findings to infants, we suggest that severe hypercapnia should be avoided, because it could cause/increase cerebrovascular injury.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Greisen, G. Autoregulation of cerebral blood flow in newborn babies. Early Hum. Dev. 81, 423–428 (2005).
Kaiser, J. R., Gauss, C. H. & Williams, D. K. The effects of hypercapnia on cerebral autoregulation in ventilated very low birth weight infants. Pediatr. Res. 58, 931–935 (2005).
Kaiser, J. R., Gauss, C. H., Pont, M. M. & Williams, D. K. Hypercapnia during the three days of life is associated with severe intraventricular hemorrhage in very low birth weight infants. J. Perinatol. 265, 279–285 (2006).
Fabres, J., Carlo, W., Phillips, V., Howard, G. & Ambalavanan, N. Both extremes of PaCO2 and the magnitude of fluctuations are associated with severe intraventricular hemorrhage in preterm infants. Pediatrics 2, 299–305 (2007).
Dobbing j and Sands, J. Comparative aspects of the brain growth spurt. Early Hum. Dev. 311, 79–83 (1979).
Buckley, N. M., Gootman, P. M., Yellin, E. L. & Brazeau, P. Age-related cardiovascular effects of catecholamines in anesthetized piglets. Circ. Res. 45, 288–292 (1979).
Pon, W. G. & Haupt, K. A. The Biology of the Pig. (Cornell University Press, Ithaca, 1978) 99.
Thome, U. H. et al. Permissive hypercapnia in extremely low birthweight infants (PHELBI): a randomized controlled multicenter trial. Lancet Respir. Med. 3, 534–543 (2015).
Kontos, H. R., Raper, A. J. & Patterson, J. L. Analysis of vasoreactivity of local pH, PCO2 and bicarbonate on pial vessels’. Stroke 8, 358–394 (1977).
Pourcyrous, M., Parfenova, H., Bada, H. S., Korones, S. B. & Leffler, C. W. Changes in cerebral cyclic nucleotides and cerebral blood flow during prolonged asphyxia and recovery in newborn pigs. Pediatr. Res. 41, 617–623 (1997).
Pryds, A., Tonnesen, J., Pryds, O., Knudsen, G. M. & Greisen, G. Cerebral pressure auto regulation and vasoreactivity in the newborn rat. Pediatr. Res. 57, 294–298 (2005).
Kety, S. S. & Schmidt, C. F. The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J. Clin. Invest. 27, 484–492 (1948).
Pryds, O. & Greisen, G. Effect of PaCO2 and Hemoglobin concentration on day to day variation of CBF in preterm neonates. Acta Pediatr. Scand. Suppl. 360, 33–36 (1989).
Pourcyrous, M. et al. Cerebrovascular responses to therapeutic dose of indomethacin in newborn pigs. Pediatr. Res. 45(4 Pt 1), 582–587 (1999).
Grubb, R. L.Jr, Raichle, M. E., Eichling, J. O. & Ter-Pogossian, M. M. The effects of changes in PaCO2 on cerebral blood volume, blood flow, and vascular mean transit time. Stroke 5, 630–639 (1974).
Bell, B. A., Foubister, G. C., Neto, N. G. & Miller, J. D. Effect of experimental common carotid arteriotomy on cerebral blood flow in rats. Neurosurgery 16, 322–326 (1985).
Pollock, J. M. et al. Hypercapnia-induced cerebral hyperperfusion: an underrecognized clinical entity. Am. J. Neuroradiol. 30, 378–385 (2009).
Warner, D. S., Turner, D. M. & Kassell, N. F. Time-dependent effects of prolonged hypercapnia on cerebrovascular parameters in dogs: acid–base chemistry. Stroke 18, 142–149 (1987).
Levasseur, J. E., Wei, E. P., Kontos, H. A. & Patterson, J. L. Jr. Responses of pial arterioles after prolonged hypercapnia and hypoxia in the awake rabbit. J. Appl. Physiol. 46, 89–95 (1979).
Lassen, N. A. Brain extracellular pH: The main factor controlling cerebral blood flow. Scand. J. Clin. Lab. Invest. 22, 247–251 (1968).
Brubakk, A. M., OH, W. & Stonestreet, B. S. Prolonged hypercapnia in the awake newborn piglet: effect on brain blood flow and cardiac output. Pediatr. Res. 21, 29–33 (1987).
Yang, S. P. & Krasney, J. A. Cerebral blood flow and metabolic responses to sustained hypercapnia in awake sheep. J. Cereb. Blood Flow. Metab. 15, 115–123 (1995).
Hino, J. K., Short, B. L., Rains-Bahrami, K. & Seale, W. R. Cerebral blood flow and metabolism during and after prolonged hypercapnia in newborn lambs. Crit. Care Med. 28, 3505–3510 (2000). 385.
Poulin, M. I., Liang, P. I. & Robbins, P. A. Dynamics of the cerebral blood flow response to step changes in end-tidal PCO2 and PO2 in humans. J. Appl. Physiol. 81, 1084–1095 (1996).
Sliwke, U., Krasney, J. A., Simon, S. G., Schmidth, P. & Noth, J. Effects of sustained low-level elevations of carbon dioxide on cerebral blood flow and auto regulation of the intracerebral arteries in humans. Aviat. Space Environ. Med. 69, 299–306 (1998).
Goldstein, B., Shannon, D. C. & Todres, I. D. Supercarbia in children: clinical course and outcome. Crit. Care Med. 18, 166–168 (1990).
Avery, M. E. et al. Is chronic lung disease in low birth weight infants preventable? a survey of eight centers. Pediatrics 79, 26–30 (1987).
Hicking, K. G., Henderson, S. J. & Jackson, R. Low mortality associated with low volume pressure limited ventilation with permissive hypercapnia in severe adult respiratory distress syndrome. Intensive Care Med. 16, 372–377 (1990).
Mariani, G., Cifuentes, J. & Carlo, W. A. Randomized trial of permissive hypercapnia in preterm infants. Pediatrics 104(5 pt 1), 1082–1088 (1999).
Ambalavanan, N. et al. PaCO2 in surfactant, positive pressure, and oxygenation randomized trial (SUPPORT). Arch. Dis. Child. Fetal Neonatal Ed. 100, F145–F149 (2015).
Vannucci, R. C., Towfighi, J., Brucklacher, R. M. & Vannucci, S. J. Effect of extreme hypercapnia on hypoxic-ischemic brain damage in the immature rat. Pediatr. Res. 49, 799–803 (2001).
Vannucci, R. C., Towfighi, J., Heltjan, D. F. & Brucklacher, R. M. Carbon dioxide protects the perinatal brain from hypoxic-ischemic damage: an experimental study in immature rats. Pediatrics 95, 868–874 (1995).
Fritz, K. I., Zubrow, A., Mishra, O. P. & Delivoria-Papadopoulos, M. Hypercapnia-induced modifications of neuronal function in cerebral cortex of newborn piglets. Pediatr. Res. 57, 299–304 (2005).
Das, S., Du, Z., Bassly, S., Singer, L. & Vicencio, A. G. Effects of chronic hypercapnia in the neonatal mouse lung and brain. Pediatr. Pumonol. 44, 176–182 (2009).
Iadecola, C. & Zhang, F. Nitric oxide-dependent and independent components of cerebrovasodilation elicited by hypercapnia. Am. J. Physiol. 266, R546–R552 (1994).
Reivich, M. E. Arterial PCO2 and cerebral hemodynamics. Am. J. Physiol. 206, 25–35 (1964).
Parfenova, H., Shibata, M., Zuckerman, S. & Leffler, C. W. CO2 and cerebral circulation in newborn pigs: cyclic nucleotides and prostanoids in vascular regulation. Am. J. Physiol. 266, H1494–H1501 (1994).
Acknowledgements
We thank Alex L. Fedinec for technical assistance and Amanda Preston, PhD for the editorial assistance. This study was supported by National Institutes of Health, Bethesda, MD RO1NS101717 (H.P.), RO1HL034059 and RO1HL42851 (C.W.L.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pourcyrous, M., Chilakala, S., Elabiad, M.T. et al. Does prolonged severe hypercapnia interfere with normal cerebrovascular function in piglets?. Pediatr Res 84, 290–295 (2018). https://doi.org/10.1038/s41390-018-0061-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-018-0061-5
This article is cited by
-
The effects of sodium bicarbonate infusion on cerebrovascular function in newborn pigs
Pediatric Research (2022)