Abstract
Background
Extrauterine life is an important factor when considering brain maturation. Few studies have investigated the development of visual evoked potentials (VEP) in extremely preterm infants, and only a minority have taken into consideration the impact of extrauterine life. The aim of this study was to assess the normal maturation of VEP in infants born prior to 29 weeks gestational age (GA) and to explore the potential influence of extrauterine life.
Methods
VEP were prospectively recorded in extremely preterm infants, and principal peaks (N0, N1, P1, N2, P2, N3) were identified. The mean of peak-time and percentages of peak appearances were assessed for three GA groups (23/24, 25/26, 27/28 weeks) and four subgroups of increasing postnatal age (PNA), up to 8 weeks after birth.
Results
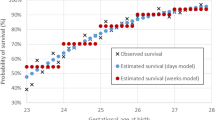
A total of 163 VEP recordings in 38 preterm infants were analyzed. With increasing GA at birth, peak-times decreased. When comparing infants with equal GA but longer extrauterine life, those with the highest PNA demonstrated the shortest VEP peak-times. However, this effect was less present in infants born prior to 25 weeks GA.
Conclusion
Provided that a certain maturational threshold is reached, extrauterine life appears to accelerate maturation of the visual system in preterm infants.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Chayasirisobhon, S. et al. Evaluation of maturation and function of visual pathways in neonates: role of flash visual-evoked potentials revisited. Clin. EEG Neurosci. 43, 18–22 (2012).
Kraemer, M., Abrahamsson, M. & Sjostrom, A. The neonatal development of the light flash visual evoked potential. Doc. Ophthalmol. 99, 21–39 (1999).
Pike, A. A., Marlow, N. & Reber, C. Maturation of the flash visual evoked potential in preterm infants. Early Hum. Dev. 54, 215–222 (1999).
Taylor, M. J., Saliba, E. & Laugier, J. Use of evoked potentials in preterm neonates. Arch. Dis. Child Fetal Neonatal Ed. 74, F70–F76 (1996).
Tsuneishi, S. Evaluation of the developing human visual system using flash-visual evoked potential. No Hattatsu 34, 141–146 (2002).
Roy, M. S., Barsoum-Homsy, M., Orquin, J. & Benoit, J. Maturation of binocular pattern visual evoked potentials in normal full-term and preterm infants from 1 to 6 months of age. Pediatr. Res. 37, 140–144 (1995).
Mirabella, G., Kjaer, P. K., Norcia, A. M., Good, W. V. & Madan, A. Visual development in very low birth weight infants. Pediatr. Res. 60, 435–439 (2006).
Woods, J. R. Jr & Plessinger, M. A. The fetal visual evoked potential. Pediatr. Res. 20, 351–355 (1986).
Klebermass-Schrehof, K. et al. Can neurophysiological assessment improve timing of intervention in posthaemorrhagic ventricular dilatation? Arch. Dis. Child Fetal Neonatal Ed. 98, F291–F297 (2013).
Taylor, M. J., Murphy, W. J. & Whyte, H. E. Prognostic reliability of somatosensory and visual evoked potentials of asphyxiated term infants. Dev. Med. Child Neurol. 34, 507–515 (1992).
Whyte, H. E. Visual-evoked potentials in neonates following asphyxia. Clin. Perinatol. 20, 451–461 (1993).
Feng, J. J., Wang, W. P., Guo, S. J., Liu, Z. W. & Xu, X. Flash visual evoked potentials in preterm infants. Ophthalmology 120, 489–494 (2013).
Tsuneishi, S. & Casaer, P. Stepwise decrease in VEP latencies and the process of myelination in the human visual pathway. Brain Dev. 19, 547–551 (1997).
Tsuneishi, S., Casaer, P., Fock, J. M. & Hirano, S. Establishment of normal values for flash visual evoked potentials (VEPs) in preterm infants: a longitudinal study with special reference to two components of the N1 wave. Electroencephalogr. Clin. Neurophysiol. 96, 291–299 (1995).
Pineda, R. G. et al. Alterations in brain structure and neurodevelopmental outcome in preterm infants hospitalized in different neonatal intensive care unit environments. J. Pediatr. 164, 52–60.e2 (2014).
Healy, E. et al. Preterm birth and adolescent social functioning-alterations in emotion-processing brain areas. J. Pediatr. 163, 1596–1604 (2013).
Smith, G. C. et al. Neonatal intensive care unit stress is associated with brain development in preterm infants. Ann. Neurol. 70, 541–549 (2011).
Klebermass, K. et al. Intra- and extrauterine maturation of amplitude-integrated electroencephalographic activity in preterm infants younger than 30 weeks of gestation. Biol. Neonate 89, 120–125 (2006).
Odom, J. V. et al. ISCEV standard for clinical visual evoked potentials (2009 update). Doc. Ophthalmol. 120, 111–119 (2010).
Schomer, D. & Lopes da Silva, F. (eds) Niedermeyer’s Electroencephalography 6th edn (Lippincott Williams & Wilkins, Phildelphia, PA, 2012).
Bayley, N. Bayley Scales of Infant Development II (Psychological Corp, San Antonio, TX, 1993).
Reuner, G. R. J., Buschmann, A., Bach, C. & Pietz, J. German version and standardization of the Bayley Scales of infant and toddler development, 3rd edition: first results on clinical validity and criteria validity of the cognitive scale and language scale. Neuropediatrics 46(S01), FV01 (2015).
Rosenbaum, P. L., Palisano, R. J., Bartlett, D. J., Galuppi, B. E. & Russell, D. J. Development of the Gross Motor Function Classification System for cerebral palsy. Dev. Med. Child Neurol. 50, 249–253 (2008).
Taylor, M. J., Menzies, R., MacMillan, L. J. & Whyte, H. E. VEPs in normal full-term and premature neonates: longitudinal versus cross-sectional data. Electroencephalogr. Clin. Neurophysiol. 68, 20–27 (1987).
O’Reilly, M. et al. Ophthalmological, cognitive, electrophysiological and MRI assessment of visual processing in preterm children without major neuromotor impairment. Dev. Sci. 13, 692–705 (2010).
Inder, T. E., Warfield, S. K., Wang, H., Huppi, P. S. & Volpe, J. J. Abnormal cerebral structure is present at term in premature infants. Pediatrics 115, 286–294 (2005).
Soubasi, V. et al. The influence of extrauterine life on the aEEG maturation in normal preterm infants. Early Hum. Dev. 85, 761–765 (2009).
Penn, A. S. C. Principles of Endogenous and Sensory Activity-Dependent Brain Development: The Visual System. The Newborn Brain 147–161 (Cambridge University Press, Cambridge, 2010).
Penn, A. A. Early brain wiring: activity-dependent processes. Schizophr. Bull. 27, 337–347 (2001).
Fulford, J. et al. Fetal brain activity in response to a visual stimulus. Hum. Brain Mapp. 20, 239–245 (2003).
Tsuneishi, S. & Casaer, P. Effects of preterm extrauterine visual experience on the development of the human visual system: a flash VEP study. Dev. Med. Child Neurol. 42, 663–668 (2000).
Tremblay, E. et al. Delayed early primary visual pathway development in premature infants: high density electrophysiological evidence. PLoS ONE 9, e107992 (2014).
Jando, G. et al. Early-onset binocularity in preterm infants reveals experience-dependent visual development in humans. Proc. Natl Acad. Sci. USA 109, 11049–11052 (2012).
Madan, A., Jan, J. E. & Good, W. V. Visual development in preterm infants. Dev. Med. Child Neurol. 47, 276–280 (2005).
Broring, T., Oostrom, K. J., Lafeber, H. N., Jansma, E. P. & Oosterlaan, J. Sensory modulation in preterm children: theoretical perspective and systematic review. PLoS ONE 12, e0170828 (2017).
Scherjon, S., Briet, J., Oosting, H. & Kok, J. The discrepancy between maturation of visual-evoked potentials and cognitive outcome at five years in very preterm infants with and without hemodynamic signs of fetal brain-sparing. Pediatrics 105, 385–391 (2000).
Whyte, H. E., Pearce, J. M. & Taylor, M. J. Changes in the VEP in preterm neonates with arousal states, as assessed by EEG monitoring. Electroencephalogr. Clin. Neurophysiol. 68, 223–225 (1987).
Bauer, I. et al. Omega-3 fatty acids modify human cortical visual processing—a double-blind, crossover study. PLoS ONE 6, e28214 (2011).
Faldella, G. et al. Visual evoked potentials and dietary long chain polyunsaturated fatty acids in preterm infants. Arch. Dis. Child Fetal Neonatal Ed. 75, F108–F112 (1996).
Kothari, R., Singh, R., Singh, S. & Bokariya, P. Effect of head circumference on parameters of pattern reversal visual evoked potential in healthy adults of central India. Nepal Med. Coll. J. 14, 75–79 (2012).
Malcolm, C. A., McCulloch, D. L. & Shepherd, A. J. Pattern-reversal visual evoked potentials in infants: gender differences during early visual maturation. Dev. Med. Child Neurol. 44, 345–351 (2002).
Sharma, R., Joshi, S., Singh, K. D. & Kumar, A. Visual evoked potentials: normative values and gender differences. J. Clin. Diagn. Res. 9, CC12–CC15 (2015).
Sanchez Fernandez, I., Loddenkemper, T., Peters, J. M. & Kothare, S. V. Electrical status epilepticus in sleep: clinical presentation and pathophysiology. Pediatr. Neurol. 47, 390–410 (2012).
Duerksen, K., Barlow, W. E. & Stasior, O. G. Fused eyelids in premature infants. Ophthal. Plast. Reconstr. Surg. 10, 234–240 (1994).
Acknowledgements
We are very grateful to all patients and parents for participating in this study, and we thank Aner Gurvitz, who advised us in English writing and critically revised the manuscript. This work was supported by the OeNB (Oesterreichische Nationalbank Anniversary Fund Nr. 13232). The sponsor was not involved in any part of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Schwindt, E., Giordano, V., Rona, Z. et al. The impact of extrauterine life on visual maturation in extremely preterm born infants. Pediatr Res 84, 403–410 (2018). https://doi.org/10.1038/s41390-018-0084-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-018-0084-y
This article is cited by
-
Visual pathway function in adults born preterm with very low birth weight: a two-country birth cohort study
Documenta Ophthalmologica (2025)