Abstract
Background
Supplementation of formula with bovine milk fat globule membranes has been shown to narrow the gap in immunological and cognitive development between breast-fed and formula-fed infants.
Method
In a double-blinded randomized controlled trial 160 formula-fed infants received an experimental formula (EF), supplemented with bovine milk fat globule membranes, or standard formula until 6 months of age. A breast-fed reference group was recruited. Lipidomic analyses were performed on plasma and erythrocyte membranes at 6 months and on serum at 4 and 12 months of age.
Results
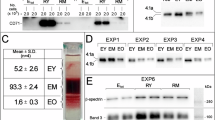
At 6 months of age, we observed a significant separation in the plasma lipidome between the two formula groups, mostly due to differences in concentrations of sphingomyelins (SM), phosphatidylcholines (PC), and ceramides, and in the erythrocyte membrane lipidome, mostly due to SMs, PEs and PCs. Already at 4 months, a separation in the serum lipidome was evident where SMs and PCs contributed. The separation was not detected at 12 months.
Conclusions
The effect of MFGM supplementation on the lipidome is likely part of the mechanisms behind the positive cognitive and immunological effects of feeding the EF previously reported in the same study population.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Agostoni, C. et al. Breast-feeding: a commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 49, 112–125 (2009).
Anderson, J. W., Johnstone, B. M. & Remley, D. T. Breast-feeding and cognitive development: a meta-analysis. Am. J. Clin. Nutr. 70, 525–535 (1999).
Horta, B. L., Loret de Mola, C. & Victora, C. G. Breastfeeding and intelligence: a systematic review and meta-analysis. Acta Paediatr. 104, 14–19 (2015).
Ip, S., Chung, M., Raman, G., Trikalinos, T. A. & Lau, J. A summary of the Agency for Healthcare Research and Quality’s evidence report on breastfeeding in developed countries. Breastfeed. Med. 4(Suppl 1), S17–S30 (2009).
Oshida, K. et al. Effects of dietary sphingomyelin on central nervous system myelination in developing rats. Pediatr. Res. 53, 589–593 (2003).
Zeisel, S. H., Char, D. & Sheard, N. F. Choline, phosphatidylcholine and sphingomyelin in human and bovine milk and infant formulas. J. Nutr. 116, 50–58 (1986).
Gurnida, D. A., Rowan, A. M., Idjradinata, P., Muchtadi, D. & Sekarwana, N. Association of complex lipids containing gangliosides with cognitive development of 6-month-old infants. Early Hum. Dev. 88, 595–601 (2012).
Wang, B. Molecular mechanism underlying sialic acid as an essential nutrient for brain development and cognition. Adv. Nutr. 3, 465s–472s (2012).
Wang, B., McVeagh, P., Petocz, P. & Brand-Miller, J. Brain ganglioside and glycoprotein sialic acid in breastfed compared with formula-fed infants. Am. J. Clin. Nutr. 78, 1024–1029 (2003).
Claumarchirant, L., Matencio, E., Sanchez-Siles, L. M., Alegria, A. & Lagarda, M. J. Sterol composition in infant formulas and estimated intake. J. Agric. Food Chem. 63, 7245–7251 (2015).
Haque, Z. U. & Mozaffar, Z. Importance of dietary cholesterol for the maturation of mouse brain myelin. Biosci. Biotechnol. Biochem. 56, 1351–1354 (1992).
Elias, P. K., Elias, M. F., D’Agostino, R. B., Sullivan, L. M. & Wolf, P. A. Serum cholesterol and cognitive performance in the Framingham Heart Study. Psychosom. Med. 67, 24–30 (2005).
Zeisel, S. H. The fetal origins of memory: the role of dietary choline in optimal brain development. J. Pediatr. 149, S131–S136 (2006).
Koletzko, B. Human milk lipids. Ann. Nutr. Metab. 69(Suppl 2), 28–40 (2016).
Tanaka, K. et al. The pilot study: sphingomyelin-fortified milk has a positive association with the neurobehavioural development of very low birth weight infants during infancy, randomized control trial. Brain. Dev. 35, 45–52 (2013).
Liao, Y., Alvarado, R., Phinney, B. & Lonnerdal, B. Proteomic characterization of human milk fat globule membrane proteins during a 12 month lactation period. J. Proteome Res. 10, 3530–3541 (2011).
King, J. C. et al. A double-blind, placebo-controlled, pilot study of bovine lactoferrin supplementation in bottle-fed infants. J. Pediatr. Gastroenterol. Nutr. 44, 245–251 (2007).
Liu, B. & Newburg, D. S. Human milk glycoproteins protect infants against human pathogens. Breastfeed. Med. 8, 354–362 (2013).
Fuller, K. L., Kuhlenschmidt, T. B., Kuhlenschmidt, M. S., Jimenez-Flores, R. & Donovan, S. M. Milk fat globule membrane isolated from buttermilk or whey cream and their lipid components inhibit infectivity of rotavirus in vitro. J. Dairy Sci. 96, 3488–3497 (2013).
Timby, N., Domellof, E., Hernell, O., Lonnerdal, B. & Domellof, M. Neurodevelopment, nutrition, and growth until 12 mo of age in infants fed a low-energy, low-protein formula supplemented with bovine milk fat globule membranes: a randomized controlled trial. Am. J. Clin. Nutr. 99, 860–868 (2014).
Timby, N. et al. Infections in infants fed formula supplemented with bovine milk fat globule membranes. J. Pediatr. Gastroenterol. Nutr. 60, 384–389 (2015).
Folch, J., Lees, M. & Sloane Stanley, G. H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226, 497–509 (1957).
Nygren, H., Seppanen-Laakso, T., Castillo, S., Hyotylainen, T. & Oresic, M. Liquid chromatography-mass spectrometry (LC-MS)-based lipidomics for studies of body fluids and tissues. Methods Mol. Biol. 708, 247–257 (2011).
O’Gorman, A. et al. Identification of a plasma signature of psychotic disorder in children and adolescents from the Avon Longitudinal Study of Parents and Children (ALSPAC) cohort. Transl. Psychiatry 7, e1240 (2017).
Pluskal, T., Castillo, S., Villar-Briones, A. & Oresic, M. MZmine 2: modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinforma. 11, 395 (2010).
Wheelock, A. M. & Wheelock, C. E. Trials and tribulations of ‘omics data analysis: assessing quality of SIMCA-based multivariate models using examples from pulmonary medicine. Mol. Biosyst. 9, 2589–2596 (2013).
Timby, N., Lönnerdal, B., Hernell, O. & Domellöf, M. Cardiovascular risk markers until 12 mo of age in infants fed a formula supplemented with bovine milk fat globule membranes. Pediatr. Res. 76(4), 394–400 (2014).
Acharjee, A. et al. The translation of lipid profiles to nutritional biomarkers in the study of infant metabolism. Metabolomics 13, 25 (2017).
Liu, H. et al. Early supplementation of phospholipids and gangliosides affects brain and cognitive development in neonatal piglets. J. Nutr. 144, 1903–1909 (2014).
Maceyka, M. & Spiegel, S. Sphingolipid metabolites in inflammatory disease. Nature 510, 58–67 (2014).
Arab, L. Biomarkers of fat and fatty acid intake. J. Nutr. 133(Suppl 3), 925s–932s (2003).
Mitchell, R. W., On, N. H., Del Bigio, M. R., Miller, D. W. & Hatch, G. M. Fatty acid transport protein expression in human brain and potential role in fatty acid transport across human brain microvessel endothelial cells. J. Neurochem. 117, 735–746 (2011).
Ochiai, Y. et al. The blood-brain barrier fatty acid transport protein 1 (FATP1/SLC27A1) supplies docosahexaenoic acid to the brain, and insulin facilitates transport. J. Neurochem. 141, 400–412 (2017).
Lagarde, M. et al. Lysophosphatidylcholine as a preferred carrier form of docosahexaenoic acid to the brain. J. Mol. Neurosci. 16, 201–204 (2001).
Nguyen, L. N. et al. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 509, 503–506 (2014).
Acar, N. et al. Lipid composition of the human eye: are red blood cells a good mirror of retinal and optic nerve fatty acids? PLoS ONE 7, e35102 (2012).
Sturm, A. & Dignass, A. U. Modulation of gastrointestinal wound repair and inflammation by phospholipids. Biochim. Biophys. Acta 1582, 282–288 (2002).
Shaikh, S. R., Fessler, M. B. & Gowdy, K. M. Role for phospholipid acyl chains and cholesterol in pulmonary infections and inflammation. J. Leukoc. Biol. 100, 985–997 (2016).
Beyersdorf, N. & Muller, N. Sphingomyelin breakdown in T cells: role in activation, effector functions and immunoregulation. Biol. Chem. 396, 749–758 (2015).
Matthan, N. R., Ip, B., Resteghini, N., Ausman, L. M. & Lichtenstein, A. H. Long-term fatty acid stability in human serum cholesteryl ester, triglyceride, and phospholipid fractions. J. Lipid Res. 51, 2826–2832 (2010).
Hyotylainen, T. & Oresic, M. Optimizing the lipidomics workflow for clinical studies—practical considerations. Anal. Bioanal. Chem. 407, 4973–4993 (2015).
Acknowledgements
We are grateful to the participating infants and their parents. We thank the research nurses Carina Forslund and Camilla Steinvall Lindberg, as well as the medical laboratory technologists Carina Lagerqvist for their dedicated field- and laboratory work. We also thank Hans Stenlund, Maria Ahnlund, Jonas Gullberg, and Annika Johansson at Swedish Metabolomics Centre (Cooperation between Umeå University and Swedish University of Agricultural Sciences, Umeå, Sweden) for plasma and erythrocyte membrane lipidomic analyses, Anette Untermann for technical assistance in the serum lipidomic analyses performed at the Steno Diabetes Center Copenhagen (Copenhagen, Denmark) and BILS (Bioinformatics for life sciences Sweden, currently NBIS) for support through Rui Climaco Pinto (based at CLiC -Computational life science cluster, Umeå University, Umeå, Sweden) for statistical advice. T.G. and N.T. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The study was funded by grants from Sweden's Innovation Agency (Vinova), Semper/Hero and Västerbotten County Council.
Author information
Authors and Affiliations
Contributions
Substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; T.G., T.S.D., L.A., M.D., O.H., T.H., M.K., B.L., M.O. and N.T. Drafting the article or revising it critically for important intellectual content; T.G., T.S.D., L.A., M.D., O.H., T.H., M.K., B.L., M.O. and N.T. Final approval of the version to be published T.G., T.S.D., L.A., M.D., O.H., T.H., M.K., B.L., M.O. and N.T.
Corresponding author
Ethics declarations
Competing interests
O.H. and B.L. are members of Hero and Semper scientific advisory boards. T.G., T.S.D., L.A., M.D., T.H., M.K., M.O. and N.T. declare no conflicts of interest.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Grip, T., Dyrlund, T.S., Ahonen, L. et al. Serum, plasma and erythrocyte membrane lipidomes in infants fed formula supplemented with bovine milk fat globule membranes. Pediatr Res 84, 726–732 (2018). https://doi.org/10.1038/s41390-018-0130-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-018-0130-9
This article is cited by
-
Milk phospholipid-coated lipid droplets modulate the infant gut microbiota and metabolome influencing weight gain
Microbiome (2025)
-
Metabolic phenotype of breast-fed infants, and infants fed standard formula or bovine MFGM supplemented formula: a randomized controlled trial
Scientific Reports (2019)
-
Fecal microbiome and metabolome of infants fed bovine MFGM supplemented formula or standard formula with breast-fed infants as reference: a randomized controlled trial
Scientific Reports (2019)