Abstract
Background
Premature birth is associated with ventricular remodeling, early heart failure, and altered left ventricular (LV) response to physiological stress.
Using computational cardiac magnetic resonance (CMR) imaging, we aimed to quantify preterm ventricular remodeling in the neonatal period, and explore contributory clinical factors.
Methods
Seventy-three CMR scans (34 preterm infants, 10 term controls) were performed to assess in-utero development and preterm ex-utero growth.
End-diastolic computational atlases were created for both cardiac ventricles; t statistics, linear regression modeling, and principal component analysis (PCA) were used to describe the impact of prematurity and perinatal factors on ventricular volumetrics, ventricular geometry, myocardial mass, and wall thickness.
Results
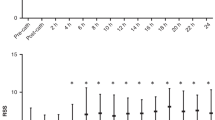
All preterm neonates demonstrated greater weight-indexed LV mass and higher weight-indexed end-diastolic volume at term-corrected age (P < 0.05 for all preterm gestations). Independent associations of increased term-corrected age LV myocardial wall thickness were (false discovery rate <0.05): degree of prematurity, antenatal glucocorticoid administration, and requirement for >48 h postnatal respiratory support.
PCA of LV geometry showed statistical differences between all preterm infants at term-corrected age and term controls.
Conclusions
Computational CMR demonstrates that significant LV remodeling occurs soon after preterm delivery and is associated with definable clinical situations. This suggests that neonatal interventions could reduce long-term cardiac dysfunction.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
World Health Organization, Media Centre. Preterm Birth. Fact sheet No. 36; Updated November 2014.
Beck, S. et al. The worldwide incidence of preterm birth: a systematic review of mortality and morbidity. Bull. World Health Organ. 88, 31–38 (2010).
Barker, D. J., Eriksson, J. G., Forsén, T. & Osmond, C. Fetal origins of adult disease: strength of effects and biological basis. Int. J. Epidemiol. 31, 1235–1239 (2002).
Centra, J. C., Roberts, G., Opie, G., Chong, J. & Doyle, L. W. Masked hypertension in extremely preterm adolescents. J. Paediatr. Child Health 51, 1060–1065 (2015).
Bassareo, P. P. & Fanos, V. Editorial: cardiovascular drug therapy in paediatric age: from metabolomics to clinical practice. Curr. Med. Chem. 21, 3107 (2014).
Crump, C., Sundquist, K., Sundquist, J. & Winkleby, M. A. Gestational age at birth and mortality in young adulthood. J. Am. Med. Assoc. 306, 1233–1240 (2011).
Aye, C. Y. L. et al. Disproportionate cardiac hypertrophy during early postnatal development in infants born preterm. Pediatr. Res. 82, 36–46 (2017).
Carr, H., Cnattingius, S., Granath, F., Ludvigsson, J. F. & Edstedt Bonamy, A.-K. Preterm Birth and risk of heart failure up to early adulthood. J. Am. Coll. Cardiol. 69, 2634–2642 (2017).
Huckstep, O. J. et al. Physiological stress elicits impaired left ventricular function in preterm-born adults. J. Am. Coll. Cardiol. 71, 1347–1356 (2018).
Lewandowski, A. J. et al. Preterm heart in adult life: cardiovascular magnetic resonance reveals distinct differences in left ventricular mass, geometry, and function. Circulation 127, 197–206 (2013).
Lewandowski, A. J. et al. Right ventricular systolic dysfunction in young adults born preterm. Circulation 128, 713–720 (2013).
Lewandowski, A. J. et al. Breast milk consumption in preterm neonates and cardiac shape in adulthood. Pediatrics 138, e20160050 (2016).
Broadhouse, K. M. et al. Cardiovascular magnetic resonance of cardiac function and myocardial mass in preterm infants: a preliminary study of the impact of patent ductus arteriosus. J. Cardiovasc. Magn. Reson. 16, 54 (2014).
Groves, A. M. et al. Functional cardiac MRI in preterm and term newborns. Arch. Dis. Child. Fetal Neonatal Ed. 96, F86–F91 (2011).
Price, A. N. et al. Neonatal cardiac MRI using prolonged balanced SSFP imaging at 3T with active frequency stabilization. Magn. Reson. Med. 70, 776–784 (2013).
Merchant, N. et al. A patient care system for early 3.0 Tesla magnetic resonance imaging of very low birth weight infants. Early Hum. Dev. 85, 779–783 (2009).
Heiberg, E. et al. Design and validation of segment – a freely available software for cardiovascular image analysis. BMC Med. Imaging 10, 1 (2010).
Yushkevich, P. A. et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31, 1116–1128 (2006).
Rueckert, D. et al. Non-rigid registration using free-form deformations: application to breast MR images. IEEE Trans. Med. Imaging 18, 712–721 (1999).
Schnabel J.A. et al. A Generic Framework for Non-rigid Registration Based on Non-uniform Multilevel Free-Form Deformations. In: Niessen W.J., Viergever M.A. (eds.) Medical Image Computing and Computer-Assisted Intervention – MICCAI 2001. MICCAI 2001. Lecture Notes in Computer Science, Vol 2208, 573–581. Springer, Berlin, Heidelberg (2001).
Studholme, C., Hill, D. L. G. & Hawkes, D. J. An overlap invariant entropy measure of 3D medical image alignment. Pattern Recognit. 32, 71–86 (1999).
Frangi, A. F., Rueckert, D., Schnabel, J. A. & Niessen, W. J. Automatic construction of multi-object three dimensional statistical shape models: application to cardiac modeling. IEEE Trans. Med. Imaging 21, 1151–1166 (2002).
Crum, W. R., Camara, O. & Hill, D. L. G. Generalized overlap measures for evaluation and validation in medical image analysis. IEEE Trans. Med. Imaging 25, 1451–1461 (2006).
Kiebel, S.J. & Holmes, A.P. (2003) The General Linear Model in Human Brain Function. In: Frackowiak, R.S.J. et al (eds) Human Brain Function, 2nd edn. Academic Press, P 725-760. https://doi.org/10.1016/B978-0-12-264841-0.X5000-8
Janz, K. F., Dawson, J. D. & Mahoney, L. T. Predicting heart growth during puberty: the Muscatine Study. Pediatrics 105, 63 (2000).
Geelhoed, J. J. M. et al. Cardiac structures track during the first 2 years of life and are associated with fetal growth and haemodynamics: the Generation R Study. Am. Heart J. 158, 71–77 (2009).
Loral, B. H. & Carabello, B. A. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation 102, 470–479 (2000).
de Vries, W. B., van der Leij, F. R., Bakker, J. M., Kamphuis, P. J., van Oosterhout, M. F., Schipper, M. E., Smid, G. B., Bartelds, B. & van Bel, F. Alterations in adult rat heart after neonatal dexamethasone therapy. Pediatr. Res. 52, 900–906 (2002).
Paech, C., Wolf, N., Thome, U. H. & Knüpfer, M. Hypertrophic intraventricular flow obstruction after very-low-dose dexamethasone (Minidex) in preterm infants: case presentation and review of the literature. J. Perinatol. 34, 244–246 (2014).
Vimala, J., Prabhu, A., Pavithran, S. & Kumar, R. N. Hydrocortisone induced hypertrophic cardiomyopathy. Int. J. Cardiol. 150, e94–e95 (2011).
Eiby, Y. A. et al. Endogenous angiotensins and catecholamines do not reduce skin blood flow or prevent hypotension in preterm piglets. Physiol. Rep. 23, e12245 (2014). pii.
Haruki, N. et al. Comparison of acute and chronic impact of adaptive servo-ventilation on left chamber geometry and function in patients with chronic heart failure. Eur. J. Heart Fail. 13, 1140–1146 (2011).
Mann, D. L., Bogaev, R. & Buckberg, G. D. Cardiac remodeling and myocardial recovery: lost in translation? Eur. J. Heart Fail. 12, 789–796 (2010).
D’Armiento, J. Matrix metalloproteinase disruption of the extracellular matrix and cardiac dysfunction. Trends Cardiovasc. Med. 12, 97–101 (2002).
Harjai, K. J. et al. Does left ventricular shape influence clinical outcome in heart failure? Clin. Cardiol. 23, 813–819 (2000).
Koilpillai, C. et al. Relation of ventricular size and function to heart failure status and ventricular dysrhythmia in patients with severe left ventricular dysfunction. Am. J. Cardiol. 77, 606–611 (1996).
Groves, A. M. et al. Disruption of intracardiac flow patterns in the newborn infant. Pediatr. Res. 71, 380–385 (2012).
Cox, D. J. & Groves, A. M. Inotropes in preterm infants – evidence for and against. Acta Paediatr. 101, 17–23 (2012).
Takahashi, Y. et al. Postnatal left ventricular contractility in very low birth weight infants. Pediatr. Cardiol. 18, 112–117 (1997).
Bensley, J. G., Stacy, V. K., De Matteo, R., Harding, R. & Black, M. J. Cardiac remodeling as a result of pre-term: implications for future cardiovascular disease. Eur. Heart J. 31, 2058–2066 (2010).
Bertagnolli, M. et al. Activation of the cardiac renin-angiotensin system in high oxygen-exposed newborn rats: angiotensin receptor blockade prevents the developmental programming of cardiac dysfunction. Hypertension 67, 774–782 (2016).
Miyawaki, M., Okutani, T., Higuchi, R. & Yoshikawa, N. The plasma angiotensin II level increases in very low-birth weight infants with neonatal chronic lung disease. Early Hum. Dev. 84, 375–379 (2008).
Hamill, N. et al. Fetal cardiac ventricular volume, cardiac output, and ejection fraction determined with 4-dimensional ultrasound using spatiotemporal image correlation and virtual organ computer-aided analysis. Am. J. Obstet. Gynecol. 76, e1–e10 (2011).
Schulz-Menger, J. et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J. Cardiovasc. Magn. Reson. 15, 35.s (2013).
Acknowledgements
The Image Registration Toolkit was used under Academic License from Ixico Ltd. This study was supported by the Centre for the Developing Brain, King’s College London & Department of Computing, Imperial College London. Funding for the study was received from the Medical Research Council & SPARKS Children’s Medical Research charity (in the form of a Clinical Research Fellowship grant for Dr. David Cox, 2012–2015).
Author information
Authors and Affiliations
Contributions
Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data—D.J.C., W.B., A.N.P., D.R., A.D.E. and A.M.G. Drafting the article or revising it critically for important intellectual content—DJ.C., W.B., A.N.P., D.R., A.D.E. and A.M.G. Final approval of the version to be published—D.J.C., A.D.E. and A.M.G.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Cox, D.J., Bai, W., Price, A.N. et al. Ventricular remodeling in preterm infants: computational cardiac magnetic resonance atlasing shows significant early remodeling of the left ventricle. Pediatr Res 85, 807–815 (2019). https://doi.org/10.1038/s41390-018-0171-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-018-0171-0
This article is cited by
-
Utilization of integrated lung ultrasound and targeted neonatal echocardiography in preterm infant follow-up: is it feasible? Assessing value and practical challenges
European Journal of Pediatrics (2026)
-
Electrocardiogram characteristics and possible associated factors in healthy Japanese children: the Tohoku Medical Megabank Project Birth and Three-Generation Cohort Study
BMC Pediatrics (2025)
-
Respiratory distress syndrome is the poster child for neonatology
Pediatric Research (2025)
-
Reference ranges of left ventricular diastolic multimodal ultrasound parameters in stable preterm infants in the early and late neonatal intensive care admission period
Journal of Perinatology (2025)
-
Intrauterine inflammation exacerbates maladaptive remodeling of the immature myocardium after preterm birth in lambs
Pediatric Research (2022)