Abstract
Background
Reduced prenatal growth followed by rapid postnatal weight gain are risk factors for developing metabolic and cardiovascular disease. Children reared in institutions experience a similar pattern of growth restriction followed by catch-up growth after removal. We explored whether patterns of catch-up growth affect metabolic and cardiovascular outcomes in previously institutionalized adolescents.
Method
A longitudinal study of institutionalized infants randomized to care as usual (n = 68) or foster care intervention (n = 68), and never institutionalized controls (n = 127). Body mass index (BMI) was measured at baseline (20 months), 30, 42 months, and ages 8, 12, 16. At age 16, metabolic and pro-inflammatory markers were derived from blood samples.
Results
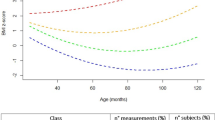
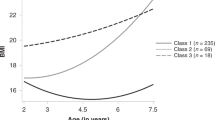
Four BMI trajectories were derived (i.e., average-stable, low-stable, elevated, and accelerated). The accelerated trajectory was comprised predominately of children randomized to foster care, who also exhibited higher levels of glycosylated hemoglobin and C-reactive protein than the other three trajectories. Also, children placed in foster care at younger ages were more likely to be on the accelerated rather than the average-stable trajectory.
Conclusions
Although catch-up growth is viewed as a positive improvement among post-institutionalized children, rapid/continuous increases in body size pose a health concern. Attention should be given to monitoring weight gain, diet, and physical activity.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Johnson, D. E. et al. Growth and associations between auxology, caregiving environment, and cognition in socially deprived Romanian children randomized to foster vs ongoing institutional care. Arch. Pedia. Adol Med. 164, 507–516 (2010).
Van IJzendoorn, M. H., Bakermans-Kranenburg, M. J. & Juffer, F. Plasticity of growth in height, weight, and head circumference: meta-analytic evidence of massive catch-up after international adoption. J. Dev. Behav. Pediatr. 28, 334–343 (2007).
Le Mare, L. & Audet, K. A longitudinal study of the physical growth and health of postinstitutionalized Romanian adoptees. Paediatr. Child Heal. 11, 85–91 (2006).
Reid, B. M. et al. Early growth faltering in post-institutionalized youth and later anthropometric and pubertal development. Pediatr. Res 82, 278–284 (2017).
Sonuga-Barke, E. J., Schlotz, W. & Rutter, M. Physical growth and maturation following early severe institutional deprivation: do they mediate specific psychopathological effects? Monogr. Soc. Res Child 75, 143–166 (2010).
Adair, L. & Cole, T. J. Rapid child growth raises blood pressure in adolescent boys who were thin at birth. Hypertension 41, 451–456 (2003).
Barker, D. J., Osmond, C., Forsén, T. J., Kajantie, E. & Eriksson, J. G. Trajectories of growth among children who have coronary events as adults. New Engl. J. Med. 353, 1802–1809 (2005).
Eriksson, J. G. et al. Catch-up growth in childhood and death from coronary heart disease: longitudinal study. BMJ 318, 427–431 (1999).
Popkin, B. M., Richards, M. K. & Montiero, C. A. Stunting is associated with overweight in children of four nations that are undergoing the nutrition transition. J. Nutr. 126, 3009–3016 (1996).
Shi, Z. et al. Early life exposure to Chinese famine modifies the association between hypertension and cardiovascular disease. J. Hypertens. 36, 54–60 (2018).
Stein, A., Zybert, P., Van de Bor, M. & Lumey, L. Intrauterine famine exposure and body proportions at birth: the Dutch Hunger Winter. Int J. Epidemiol. 33, 831–836 (2004).
Stanner, S. & Yudkin, J. Fetal programming and the Leningrad Siege study. Twin Res Hum. Genet 4, 287–292 (2001).
Ozanne, S. E. & Hales, C. N. Catch-up growth and obesity in male mice. Nature 427, 411–412 (2004).
Shin, B. C., Dai, Y., Thamotharan, M., Gibson, L. C. & Devaskar, S. U. Pre- and postnatal calorie restriction perturbs early hypothalamic neuropeptide and energy balance. J. Neurosci. Res 90, 1169–1182 (2012).
Ekelund, U. et al. Upward weight percentile crossing in infancy and early childhood independently predicts fat mass in young adults: the Stockholm Weight Development Study (SWEDES). Am. J. Clin. Nutr. 83, 324–330 (2006).
Ong, K. K. & Loos, R. J. Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr. 95, 904–908 (2006).
Baird, J. et al. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ 331, 929 (2005).
Leunissen, R. W., Kerkhof, G. F., Stijnen, T. & Hokken-Koelega, A. Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. JAMA 301, 2234–2242 (2009).
Singhal, A. et al. Nutrition in infancy and long-term risk of obesity: evidence from 2 randomized controlled trials. Am. J. Clin. Nutr. 92, 1133–1144 (2010).
Kim, C. S. et al. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J. Obes. 30, 1347–1355 (2006).
Donath, M. Y. & Shoelson, S. E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 11, 98–107 (2011).
Simmonds, M., Llewellyn, A., Owen, C. G. & Woolacott, N. Predicting adult obesity from childhood obesity: a systematic review and meta-analysis. Obes. Rev. 17, 95–107 (2015).
Zeanah, C. H. et al. Designing research to study the effects of institutionalization on brain and behavioral development: The Bucharest Early Intervention Project. Dev. Psychopathol. 15, 885–907 (2003).
de Onis, M. D. WHO child growth standards based on length/height, weight and age. Acta Paediatr. 95, 76–85 (2007).
Andersson, A., Lindh, J. & Eriksson, A. Evaluation of the HemoCue HbA1c 501 system in primary care settings. Point Care: J. Near Patient Test. Technol. 16, 128–130 (2017).
McDade, T. W. High-sensitivity enzyme immunoassay for C-reactive protein in dried blood spots. Clin. Chem. 50, 652–654 (2004).
Singer, J. D. & Willett, J. B. Applied Longitudinal Data Analysis. (Oxford University Press, New York, 2003).
Hothorn, T., Hornik, K., Wiel, M. A. & Zeileis, A. Implementing a class of permutation tests: ThecoinPackage. J. Stat. Softw. 28, 1–23 (2008).
Berends, L. M., Fernandez-Twinn, D. S., Martin-Gronert, M. S., Cripps, R. L. & Ozanne, S. E. Catch-up growth following intra-uterine growth-restriction programmes an insulin-resistant phenotype in adipose tissue. Int J. Pediatr. Obes. 37, 1051–1057 (2012).
Ong, K. K. et al. Insulin sensitivity and secretion in normal children related to size at birth, postnatal growth, and plasma insulin-like growth factor-I levels. Diabetologia 47, 1064–1070 (2004).
Warner, M. J. & Ozanne, S. E. Mechanisms involved in the developmental programming of adulthood disease. Biochem J. 427, 333–347 (2010).
Relton CL, Davey-Smith G, Ozanne SE. Developmental epigenetic programming in diabetes and obesity. In eds Jirtle R, Tyson E. Environmental Epigenomics in Health and Disease. Berlin: Springer; 2013: 235-253.
Lewis, D. S. et al. Preweaning food intake influences the adiposity of young adult baboons. J. Clin. Invest 78, 899–905 (1986).
Rueda-Clausen, C. F., Morton, J. S. & Davidge, S. T. The early origins of cardiovascular health and disease: who, when, and how. Sem Reprod Med. 29, 97–210 (2011).
Buscot, M. J. et al. Distinct child-to-adult body mass index trajectories are associated with different levels of adult cardiometabolic risk. Eur. Heart J. 39, 2263–2270 (2018).
Ford, E. S. C-reactive protein concentration and cardiovascular disease risk factors in children: findings from the National Health and Nutrition Examination Survey 1999–2000. Circulation 108, 1053–1058 (2003).
Juonala, M. Childhood C-reactive protein in predicting CRP and carotid intima-media thickness in adulthood: The Cardiovascular Risk in Young Finns Study. Arter. Throm Vas. 26, 1883–1888 (2006).
Steene-Johannessen, J., Kolle, E., Reseland, J. E., Anderssen, S. A. & Andersen, L. B. Waist circumference is related to low-grade inflammation in youth. Int J. Pediatr. Obes. 5, 313–319 (2010).
Ong, K. K. et al. Postnatal growth in preterm infants and later health outcomes: a systematic review. Acta Paediatr. 104, 974–986 (2015).
Zeanah, C. H., Humphreys, K. L., Fox, N. A. & Nelson, C. A. Alternatives for abandoned children: insights from the Bucharest Early Intervention Project. Curr. Opin. Psychol. 15, 182–188 (2017).
Funding Source
The John D. and Catherine T. MacArthur Foundation, the Binder Family Foundation, the Palix Foundation, the Jacobs Foundation, and the National Institute of Mental Health (R01MH091363) to C.A.N. and the Palix Foundation to C.H.Z..
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Tang, A., Slopen, N., Nelson, C.A. et al. Catch-up growth, metabolic, and cardiovascular risk in post-institutionalized Romanian adolescents. Pediatr Res 84, 842–848 (2018). https://doi.org/10.1038/s41390-018-0196-4
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-018-0196-4
This article is cited by
-
Effect of chorioamnionitis on postnatal growth in very preterm infants: a population-based study in Japan
Archives of Gynecology and Obstetrics (2024)
-
The association of gestational age and birthweight with blood pressure, cardiac structure, and function in 4 years old: a prospective birth cohort study
BMC Medicine (2023)
-
Catch-up growth in juvenile rats, fat expansion, and dysregulation of visceral adipose tissue
Pediatric Research (2022)
-
Liver transcriptome profiling and functional analysis of intrauterine growth restriction (IUGR) piglets reveals a genetic correction and sexual-dimorphic gene expression during postnatal development
BMC Genomics (2020)
-
Identifying typical trajectories in longitudinal data: modelling strategies and interpretations
European Journal of Epidemiology (2020)