Abstract
Study objectives
Current evidence in adults suggests that, independent of obesity, obstructive sleep apnea (OSA) can lead to autonomic dysfunction and impaired glucose metabolism, but these relationships are less clear in children. The purpose of this study was to investigate the associations among OSA, glucose metabolism, and daytime autonomic function in obese pediatric subjects.
Methods
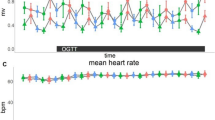
Twenty-three obese boys participated in: overnight polysomnography; a frequently sampled intravenous glucose tolerance test; and recordings of spontaneous cardiorespiratory data in both the supine (baseline) and standing (sympathetic stimulus) postures.
Results
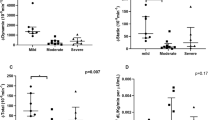
Baseline systolic blood pressure and reactivity of low-frequency heart rate variability to postural stress correlated with insulin resistance, increased fasting glucose, and reduced beta-cell function, but not OSA severity. Baroreflex sensitivity reactivity was reduced with sleep fragmentation, but only for subjects with low insulin sensitivity and/or low first-phase insulin response to glucose.
Conclusions
These findings suggest that vascular sympathetic activity impairment is more strongly affected by metabolic dysfunction than by OSA severity, while blunted vagal autonomic function associated with sleep fragmentation in OSA is enhanced when metabolic dysfunction is also present.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Martín-Timón, I., Sevillano-Collantes, C., Segura-Galindo, A. & Cañizo-Gómez, J. d. F. Type 2 diabetes and cardiovascular disease: have all risk factors the same strength? World J. Diabetes 5, 444–470 (2014).
Kohler, M. Risk factors and treatment for obstructive sleep apnea amongst obese children and adults. Curr. Opin. Allergy Clin. Immunol. 9, 4–9 (2009).
Gami, A. S. et al. Obstructive sleep apnea, obestiy, and the risk of incident atrial fibrillation. J. Am. Coll. Cardiol. 49, 565–571 (2007).
McNicholas, W. T. & Bonsignore, M. R. Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanims and research priorities. Eur. Respir. J. 29, 156–178 (2007).
Ip, M. S. M. et al. Obstructive sleep apnea is independently associated with insulin resistance. Am. J. Respir. Crit. Care Med. 165, 670–676 (2002).
Punjabi, N. M. & Polotsky, V. Y. Disorders of glucose metabolism in sleep apnea. J. Appl. Physiol. 99, 1998–2007 (2005).
Chasens, E. R., Weaver, T. E. & Umlauf, M. G. Insulin resistance and obstructive sleep apnea: is increased sympathetic stimulation the link?. Biol. Res. Nurs. 5, 87–96 (2003).
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Executive Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol Education Program (Adult Treatment Panel III). JAMA, 285, 2486–2497 (2001).
American Diabetes Association. Type 2 diabetes in children and adolescents. Pediatrics 105, 671–680 (2000).
Lesser, D. J. et al. Sleep fragmentation and intermittent hypoxemia are associated with decreased insulin sensitivity in obese adolescent Latino males. Pediatr. Res. 72, 293–298 (2012).
Shaibi, G. Q. & Goran, M. I. Examining metabolic syndrome definitions in overweight Hispanic youth: a focus on insulin resistance. J. Pediatr. 152, 171–176 (2008).
Goran, M. I., Bergman, R. N., Cruz, M. L. & Watanabe, R. Insulin resistance and associated compensatory responses in African-American and Hispanic children. Diabetes Care 25, 2184–2190 (2002).
Cruz, M. L. et al. The metabolic syndrome in overweight Hispanic youth and the role of insulin sensitivity. J. Clin. Endocrinol. Metab. 89, 108–113 (2004).
Berry, R. B. et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for scoring of sleep and associated events. J. Clin. Sleep. Med. 8, 597–619 (2012).
Witmans, M. B., Keens, T. G., Davidson Ward, S. L. & Marcus, C. L. Obstructive hypopneas in children and adolescents. Am. J. Respir. Crit. Care Med. 168, 1540 (2003).
Kang, K.-T. et al. Impacts of disease severity on postoperative complications in children with sleep-disordered breathing. Laryngoscope 127, 2646–2652 (2017).
Matthews, D. R. et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 (1985).
Galvin, P. et al. A simple method for quantitation of insulin sensitivity and insulin release from an intravenous glucose tolerance test. Diabet. Med. 9, 921–928 (1992).
Punjabi, N. M. & Beamer, B. A. Alterations in glucose disposal in sleep-disordered breathing. Am. J. Respir. Crit. Care Med. 179, 235–240 (2009).
Khoo, M. C. K., Oliveira, F. M. G. S. & Cheng, L. Understanding the metabolic syndrome: a modeling perspective. IEEE Rev. Biomed. Eng. 6, 143–155 (2013).
Bergman, R. N., Ider, Y. Z., Bowden, C. R. & Cobelli, C. Quantitative estimation of insulin sensitivity. Am. J. Physiol. 236, E667–E677 (1979).
Carnethon, M. R. et al. Does the cardiac autonomic response to postural change predict incident coronayr heart disease and mortality? The atherosclerosis risk in communities study. Am. J. Epidemiol. 155, 48–56 (2002).
Swenne, C. A. Baroreflex sensitivity: mechanisms and measurement. Neth. Heart J. 21, 58–60 (2013).
Pinna, G. D., Maestri, R., Raczak, G. & La Rovere, M. T. Measuring baroreflex sensitivity from the gain function between arterial pressure and heart period. Clin. Sci. 103, 81–88 (2002).
Goran, M. I., Shaibi, G. Q., Weigensberg, M. J., Davis, J. N. & Cruz, M. L. Deterioration of insulin sensitivity and beta-cell function in overweight Hispanic children during pubertal transition: a longitudinal assessment. Int J. Pediatr. Obes. 1, 139–145 (2006).
Hassard, T. H. Understanding Biostatistics (Mosby-Year Book, St. Louis, MO, 1991).
Chatelain, J.-B. & Ralf, K. Spurious regressions and near-multicollinearity, with an application to aid, policies and growth. J. Macroecon. 39, 85–96 (2014).
Stewart, J. M. Mechanisms of sympathetic regulation in orthostatic intolerance. J. Appl. Physiol. 113, 1659–1668 (2012).
Pagani, M. et al. Assessment of the neural control of the circulation during psychological stress. J. Auton. Nerv. Syst. 35, 33–42 (1991).
Malliani, A. et al. Spectral analysis to assess increased sympathetic tone in arterial hypertension. Hypertension 4(suppl III), 36–42 (1991).
Enright, P. L., Goodwin, J. L., Sherrill, D. L., Quan, J. R. & Quan, S. F. Blood pressure elevation associated with sleep-related breathing disorder in a community sample of white and hispanic children: the Tucson Children’s Assessment of Sleep Apnea Study. Arch. Pediatr. Adolesc. Med. 157, 901–904 (2003).
Nisbet, L. C., Yiallourou, S. R., Walter, L. M. & Horne, R. S. C. Blood pressure regulation, autonomic control and sleep disordered breathing in children. Sleep. Med. Rev. 18, 179–189 (2014).
Eyck, A. V. et al. Sleep disordered breating and autonomic function in overweight and obese children and adolescents. ERS Open Res. 2. https://doi.org/10.1183/23120541.00038-2016 (2016).
Bhushan, B. et al. Metabolic alterations in children with obstructive sleep apnea. Int J. Pediatr. Otorhinolaryngol. 78, 854–859 (2014).
Carlson, J. T., Hedner, J. A., Sellgren, J., Elam, M. & Wallin, G. Depressed baroreflex sensitivity in patients with obstructive sleep apnea. Am. J. Resp. Crit. Care Med. 154, 1490–1496 (1996).
Narkiewicz, K. et al. Baroreflex control of sympathetic nerve activity and heart rate in obstructive sleep apnea. Hypertension 32, 1039–1043 (1998).
Jo, J. A. et al. Determinants of heart rate variability in obstructive sleep apnea syndrome during wakefulness and sleep. Am. J. Physiol. Heart Circ. Physiol. 288, H1103–H1112 (2005).
Casadei, B., Meyer, T. E., Coats, A. J. S., Conway, J. & Sleight, P. Baroreflex control of stroke volume in man: an effect mediated by the vagus. Am. J. Physiol. Cell Physiol. 448, 539–550 (1992).
Koren, D., Gozal, D., Philby, M. F., Bhattacharjee, R. & Kheirandish-Gozal, L. Impact of obstructive sleep apnoea on insulin resistance in nonobese and obese children. ERJ Express 52. https://doi.org/10.1183/13993003.01430-2015, 2015.
Cerutti, F. et al. Impairment in cardiovascular autonomic pattern in obese adolescents with Type 2 diabetes mellitus. J. Endocrinol. Invest. 33, 539–543 (2010).
Peltier, A. C. et al. Autonomic dysfunction in obstructive sleep apnea is associated with impaired glucose regulation. Sleep. Med. 8, 149–155 (2007).
Wang, W., Redline, S. & Khoo, M. C. K. Autonomic markers of impaired glucose metabolism: effects of sleep disordered breathing. J. Diabetes Sci. Technol. 6, 1–13 (2012).
Berne, C., Fagius, J., Pollare, T. & Hjemdahl, P. The sympathetic response to euglycaemic hyperinsulinaemia: the evidence from microelectrode nerve recordings in healthy subjects. Diabetologia 35, 873–879 (1992).
Anderson, E. A., Balon, T. W., Hoffman, R. P., Sinkey, C. A. & Mark, A. L. Insulin increases sympathetic activity but not blood pressure in borderline hypertensive humans. Hypertension 19, 621–627 (1992).
Reaven, G. M. Relationship between insulin resistance and hypertension. Diabetes Care 14, 33–38 (1991).
Funding
This work was supported in part by NIH Grants HL105210, HL090451, EB001978, RR00047, and the USC Center for Transdisciplinary Research on Energetics and Cancer (TREC U54 CA 116848). F.M.S. Oliveira also thanks the Fulbright/CAPES program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Oliveira, F.M.S., Tran, W.H., Lesser, D.J. et al. Abnormalities in autonomic function in obese boys at-risk for insulin resistance and obstructive sleep apnea. Pediatr Res 85, 790–798 (2019). https://doi.org/10.1038/s41390-018-0226-2
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-018-0226-2
This article is cited by
-
Poverty and chronic illness: why safety net programs matter
Pediatric Research (2019)