Abstract
Background
There are few and conflicting data on the role of cytochrome P450 2D6 (CYP2D6) polymorphisms in relation to risperidone adverse events (AEs) in children. This study assessed the association between CYP2D6 metabolizer status and risk for risperidone AEs in children.
Methods
Children ≤18 years with at least 4 weeks of risperidone exposure were identified using BioVU, a de-identified DNA biobank linked to electronic health record data. The primary outcome of this study was AEs. After DNA sequencing, individuals were classified as CYP2D6 poor, intermediate, normal, or ultrarapid CYP2D6 metabolizers.
Results
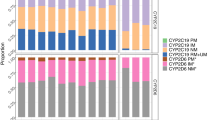
For analysis, the 257 individuals were grouped as poor/intermediate metabolizers (n = 33, 13%) and normal/ultrarapid metabolizers (n = 224, 87%). AEs were more common in poor/intermediate vs. normal/ultrarapid metabolizers (15/33, 46% vs. 61/224, 27%, P = 0.04). In multivariate analysis adjusting for age, sex, race, and initial dose, poor/intermediate metabolizers had increased AE risk (adjusted odds ratio 2.4, 95% confidence interval 1.1–5.1, P = 0.03).
Conclusion
Children with CYP2D6 poor or intermediate metabolizer phenotypes are at greater risk for risperidone AEs. Pre-prescription genotyping could identify this high-risk subset for an alternate therapy, risperidone dose reduction, and/or increased monitoring for AEs.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Dean, L. In Medical Genetics Summaries (eds Pratt, V. et al.) (National Center for Biotechnology Information (US), Bethesda, 2012). http://www.ncbi.nlm.nih.gov/books/NBK425795/.
Food and Drug Administration. Label for Risperdal (Ortho-McNeil-Janssen Pharmaceuticals, Inc., Titusville, 2007). https://www.accessdata.fda.gov/drugsatfda_docs/labe/2009/020272s056,020588s044,021346s033,021444s03lbl.pdf.
Harrison, J. N., Cluxton-Keller, F. & Gross, D. Antipsychotic medication prescribing trends in children and adolescents. J. Pediatr. Health Care 26, 139–145 (2012).
Aka, I. et al. Clinical pharmacogenetics of cytochrome P450-associated drugs in children. J. Pers. Med. 7, 14 (2017).
De Hert, M., Dobbelaere, M., Sheridan, E. M., Cohen, D. & Correll, C. U. Metabolic and endocrine adverse effects of second-generation antipsychotics in children and adolescents: a systematic review of randomized, placebo controlled trials and guidelines for clinical practice. Eur. Psychiatry 26, 144–158 (2011).
Leckman-Westin, E. et al. Differences in Medicaid antipsychotic medication measures among children with SSI, foster care, and income-based aid. J. Manag Care Spec. Pharm. 24, 238–246 (2018).
Correll, C. U. et al. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. J. Am. Med. Assoc. 302, 1765–1773 (2009).
Correll, C. U. et al. Recognizing and monitoring adverse events of second-generation antipsychotics in children and adolescents. Child Adolesc. Psychiatr. Clin. N. Am. 15, 177–206 (2006).
Germann, D., Kurylo, N. & Han, F. In Profiles of Drug Substances, Excipients and Related Methodology (ed. Brittain, H. G.) 313–361 (Academic Press, New York, 2012). http://www.sciencedirect.com/science/article/pii/B9780123972200000088.
Gaedigk, A. Complexities of CYP2D6 gene analysis and interpretation. Int. Rev. Psychiatry 25, 534–553 (2013).
Hicks, J. K., Swen, J. J. & Gaedigk, A. Challenges in CYP2D6 phenotype assignment from genotype data: a critical assessment and call for standardization. Curr. Drug Metab. 15, 218–232 (2014).
Gaedigk, A., Sangkuhl, K., Whirl-Carrillo, M., Klein, T. & Leeder, J. S. Prediction of CYP2D6 phenotype from genotype across world populations. Genet. Med. 19, 69–76 (2017).
de Leon, J. et al. The CYP2D6 poor metabolizer phenotype may be associated with risperidone adverse drug reactions and discontinuation. J. Clin. Psychiatry 66, 15–27 (2005).
Cabaleiro, T. et al. Effect of polymorphisms on the pharmacokinetics, pharmacodynamics, and safety of risperidone in healthy volunteers. Hum. Psychopharmacol. 29, 459–469 (2014).
Puangpetch, A. et al. CYP2D6 polymorphisms and their influence on risperidone treatment. Pharm. Pers. Med. 9, 131–147 (2016).
Novalbos, J. et al. Effects of CYP2D6 genotype on the pharmacokinetics, pharmacodynamics, and safety of risperidone in healthy volunteers. J. Clin. Psychopharmacol. 30, 504–511 (2010).
Dodgen, T. M. et al. Risperidone-associated adverse drug reactions and CYP2D6 polymorphisms in a South African cohort. Appl. Transl. Genom. 5, 40–46 (2015).
Correia, C. T. et al. Pharmacogenetics of risperidone therapy in autism: association analysis of eight candidate genes with drug efficacy and adverse drug reactions. Pharm. J. 10, 418–430 (2010).
Troost, P. W. et al. Prolactin release in children treated with risperidone: impact and role of CYP2D6 metabolism. J. Clin. Psychopharmacol. 27, 52–57 (2007).
Youngster, I. et al. CYP2D6 genotyping in paediatric patients with autism treated with risperidone: a preliminary cohort study. Dev. Med. Child Neurol. 56, 990–994 (2014).
Vanwong, N. et al. Impact of CYP2D6 polymorphism on steady-state plasma levels of risperidone and 9-hydroxyrisperidone in Thai children and adolescents with autism spectrum disorder. J. Child Adolesc. Psychopharmacol. 27, 185–191 (2016).
Bowton, E. et al. Biobanks and electronic medical records: enabling cost-effective research. Sci. Transl. Med. 6, 234cm3 (2014).
Roden, D. M. et al. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin. Pharmacol. Ther. 84, 362–369 (2008).
McGregor, T. L. et al. Inclusion of pediatric samples in an opt-out biorepository linking DNA to de-identified medical records: pediatric BioVU. Clin. Pharmacol. Ther. 93, 204–211 (2013).
Aman, M. G. et al. Acute and long-term safety and tolerability of risperidone in children with autism. J. Child Adolesc. Psychopharmacol. 15, 869–884 (2005).
Haas, M., Karcher, K. & Pandina, G. J. Treating disruptive behavior disorders with risperidone: a 1-year, open-label safety study in children and adolescents. J. Child Adolesc. Psychopharmacol. 18, 337–345 (2008).
Findling, R. L. et al. A double-blind pilot study of risperidone in the treatment of conduct disorder. J. Am. Acad. Child Adolesc. Psychiatry 39, 509–516 (2000).
Flockhart, D. Flockhart Table—Drug Interactions: Cytochrome P450 Drug Interaction Table (Indiana University School of Medicine, 2007). https://drug-interactions.medicine.iu.edu/Main-Table.aspx.
Crews, K. R. et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for codeine therapy in the context of cytochrome P450 2D6 (CYP2D6) genotype. Clin. Pharmacol. Ther. 91, 321–326 (2012).
Crews, K. R. et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for cytochrome P450 2D6 genotype and codeine Therapy. Clin. Pharmacol. Ther. 95, 376–382 (2014).
Nussbaum, L. A. et al. Molecular study of weight gain related to atypical antipsychotics: clinical implications of the CYP2D6 genotype. Rom. J. Morphol. Embryol. 55, 877–884 (2014).
Dos Santos-Júnior, A. et al. Pharmacogenetics of risperidone and cardiovascular risk in children and adolescents. Int. J. Endocrinol. Article ID5872423 (2016).
PharmGKB. Gene-Specif. Inf. Tables CYP2D6 (2018). https://www.pharmgkb.org/page/cyp2d6RefMaterials.
CPIC. Guidelines (2018). https://cpicpgx.org/guidelines/.
Correll, C. U. Assessing and maximizing the safety and tolerability of antipsychotics used in the treatment of children and adolescents. J. Clin. Psychiatry 69, 26–36 (2008).
Hoekstra, P. J. et al. Risperidone-induced weight gain in referred children with autism spectrum disorders is associated with a common polymorphism in the 5-hydroxytryptamine 2C receptor gene. J. Child Adolesc. Psychopharmacol. 20, 473–477 (2010).
King, B., Zwi, K., Nunn, K., Longworth, J. & Dossetor, D. Use of risperidone in a paediatric population: an observational study. J. Paediatr. Child Health 39, 523–527 (2003).
Calarge, C. A. & Miller, D. D. Predictors of risperidone and 9-hydroxyrisperidone serum concentration in children and adolescents. J. Child Adolesc. Psychopharmacol. 21, 163–169 (2011).
Pediatric and Neonatal Lexi-Drugs Online. In Lexicomp Online (Wolters Kluwer Clinical Drug Information, Inc., Hudson). https://www.uptodate.com/contents/risperidone-pediatric-drug-information.
Bates, D. W. et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA 274, 29–34 (1995).
Acknowledgements
Data extraction and formatting was performed by Christian Shaffer, Vanderbilt University Medical Center. This work used a dataset from Vanderbilt University Medical Center’s BioVU, which is supported by institutional funding and by the CTSA grant UL1 TR000445 from NIH/NCATS. This work was also supported by NIH/NCATS KL2 TR000446 (S.L.V.D.), Burroughs Wellcome Fund IRSA 1015006 (S.L.V.D.), and the NIH/NIGMS Clinical Pharmacology Training Program 5T32 GM007569 (K.A.O. and K.M.N.).
Author information
Authors and Affiliations
Contributions
K.A.O. conceptualized and designed the study, designed the data collection instruments, collected the data, interpreted the data, and revised the manuscript. K.M.N. analyzed and interpreted the data, drafted the initial manuscript, and revised the manuscript. R.J.C. collected the data and revised the manuscript. I.T.A. analyzed the data and revised the manuscript. A.C.M.-H. revised the manuscript. D.M.R. designed the study and revised the manuscript. S.L.V.D. conceptualized and designed the study, reviewed the data collection instruments, coordinated and supervised data collection, and revised the manuscript. All authors gave final approval of this version of the article.
Corresponding author
Ethics declarations
Competing interests
S.L.V.D. has been an invited speaker to Merck. The other authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Oshikoya, K.A., Neely, K.M., Carroll, R.J. et al. CYP2D6 genotype and adverse events to risperidone in children and adolescents. Pediatr Res 85, 602–606 (2019). https://doi.org/10.1038/s41390-019-0305-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-019-0305-z
This article is cited by
-
The Frequency of CYP2D6 and CYP3A4/5 Genotypes and The Impact of Their Allele Translation and Phenoconversion-Predicted Enzyme Activity on Risperidone Pharmacokinetics in Saudi Children with Autism
Biochemical Genetics (2024)
-
The polymorphisms of candidate pharmacokinetic and pharmacodynamic genes and their pharmacogenetic impacts on the effectiveness of risperidone maintenance therapy among Saudi children with autism
European Journal of Clinical Pharmacology (2024)