Abstract
Background
Protein-losing enteropathy (PLE) is a severe complication of Fontan circulation with increased risk of end-organ dysfunction. We evaluated tissue oxygenation via near-infrared spectroscopy (NIRS) at different exercise levels in Fontan patients.
Methods
Assessment of multisite NIRS during cycle ergometer exercise and daily activities in three groups: Fontan patients with PLE; without PLE; patients with dextro-transposition of the great arteries (d-TGA); comparing univentricular with biventricular circulation and Fontan with/without PLE. Renal threshold analysis (<65%;<55%;<45%) of regional oxygen saturation (rSO2) was performed.
Results
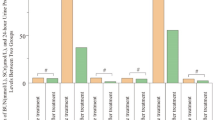
Fontan patients showed reduced rSO2 (p < 0.05) in their quadriceps femoris muscle compared with biventricular d-TGA patients at all time points. rSO2 in renal tissue was reduced at baseline (p = 0.002), exercise (p = 0.0062), and daily activities (p = 0.03) in Fontan patients with PLE. Renal threshold analysis identified critically low renal rSO2 (rSO2 < 65%) in Fontan patients with PLE during exercise (95% of monitoring time below threshold) and daily activities (83.7% time below threshold).
Conclusion
Fontan circulation is associated with decreased rSO2 values in skeletal muscle and hypoxemia of renal tissue solely in patients with PLE. Reduced rSO2 already during activities of daily life, might contribute to comorbidities in patients with Fontan circulation, including PLE and renal failure.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Fontan, F. & Baudet, E. Surgical repair of tricuspid atresia. Thorax 26, 240–248 (1971).
Khairy, P. et al. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation 117, 85–92 (2008).
Wilson, T. G. et al. Twenty-five year outcomes of the lateral tunnel Fontan procedure. Semin. Thorac. Cardiovasc. Surg. 29, 347–353 (2017).
Downing, T. E. et al. Long-term survival after the Fontan operation: twenty years of experience at a single center. J. Thorac. Cardiovasc. Surg. 154, 243–253.e2 (2017).
Pundi, K. N. et al. 40-Year follow-up after the fontan operation: long-term outcomes of 1,052 patients. J. Am. Coll. Cardiol. 66, 1700–1710 (2015).
Gewillig, M. & Brown, S. C. The Fontan circulation after 45 years: update in physiology. Heart 102, 1081–1086 (2016).
Gewillig, M. et al. The Fontan circulation: who controls cardiac output? Interact. Cardiovasc. Thorac. Surg. 10, 428–433 (2010).
Rychik, J. The relentless effects of the Fontan paradox. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 19, 37–43 (2016).
Alsaied, T. et al. Factors associated with long-term mortality after Fontan procedures: a systematic review. Heart 103, 104–110 (2017).
Mori, M. et al. Beyond a broken heart: circulatory dysfunction in the failing Fontan. Pediatr. Cardiol. 35, 569–579 (2014).
Mizuno, M. et al. Diverse multi-organ histopathologic changes in a failed Fontan patient. Pediatr. Int. 58, 1061–1065 (2016).
Fleck, T. et al. Propofol effect on cerebral oxygenation in children with congenital heart disease. Pediatr. Cardiol. 36, 543–549 (2015).
Loomba, R. S. et al. Effect of Fontan fenestration on regional venous oxygen saturation during exercise: further insights into Fontan fenestration closure. Pediatr. Cardiol. 35, 514–520 (2014).
Hoffman, G. M. et al. Perioperative cerebral oxygen saturation in neonates with hypoplastic left heart syndrome and childhood neurodevelopmental outcome. J. Thorac. Cardiovasc. Surg. 146, 1153–1164 (2013).
Rao, R. P. et al. Measurement of regional tissue bed venous weighted oximetric trends during exercise by near infrared spectroscopy. Pediatr. Cardiol. 30, 465–471 (2009).
Rao, R. P. et al. Cerebral hemodynamics in the presence of decreased systemic venous compliance in patients with Fontan physiology may limit anaerobic exercise capacity. Pediatr. Cardiol. 31, 208–214 (2010).
Forman, E. et al. Noninvasive continuous cardiac output and cerebral perfusion monitoring in term infants with neonatal encephalopathy: assessment of feasibility and reliability. Pediatr. Res. 82, 789–795 (2017).
Ruf, B. et al. Intraoperative renal near-infrared spectroscopy indicates developing acute kidney injury in infants undergoing cardiac surgery with cardiopulmonary bypass: a case-control study. Crit. Care 19, 27 (2015).
Su, X. W. et al. Improved cerebral oxygen saturation and blood flow pulsatility with pulsatile perfusion during pediatric cardiopulmonary bypass. Pediatr. Res. 70, 181–185 (2011).
Navaratnam, D. et al. Exercise-induced systemic venous hypertension in the Fontan circulation. Am. J. Cardiol. 117, 1667–1671 (2016).
Kromeyer-Hauschild, K. et al. Perzentile für den body-mass-Index für das Kindes- und Jugendalter unter Heranziehung verschiedener deutscher Stichproben. Mon. Kinderheilkd. 149, 807–818 (2001).
Horibata, Y., Murakami, T. & Niwa, K. Effect of the oral vasopressin receptor antagonist tolvaptan on congestive cardiac failure in a child with restrictive cardiomyopathy. Cardiol. Young-. 24, 155–157 (2014).
Bernal, N. P. et al. Cerebral and somatic near-infrared spectroscopy in normal newborns. J. Pediatr. Surg. 45, 1306–1310 (2010).
Colasacco, C. et al. Near-infrared spectroscopy monitoring to predict postoperative renal insufficiency following repair of congenital heart disease. World J. Pediatr. Congenit. Heart Surg. 2, 536–540 (2011).
Choi, D. K. et al. Intraoperative renal regional oxygen desaturation can be a predictor for acute kidney injury after cardiac surgery. J. Cardiothorac. Vasc. Anesth. 28, 564–571 (2014).
Dent, C. L. et al. Brain magnetic resonance imaging abnormalities after the Norwood procedure using regional cerebral perfusion. J. Thorac. Cardiovasc. Surg. 130, 1523–1530 (2005).
Faul, F. et al. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav. Res. Methods 41, 1149–1160 (2009).
Faul, F. et al. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191 (2007).
Callewaert, M. et al. Quadriceps muscle fatigue in trained and untrained boys. Int. J. Sports Med. 34, 14–20 (2013).
Bowen, T. S. et al. The spatial distribution of absolute skeletal muscle deoxygenation during ramp-incremental exercise is not influenced by hypoxia. Adv. Exp. Med. Biol. 876, 19–26 (2016).
Okushima, D. et al. Muscle deoxygenation in the quadriceps during ramp incremental cycling: deep vs. superficial heterogeneity. J. Appl. Physiol. 2015, 1313–1319 (1985).
Hager, A. et al. Predictors of sildenafil effects on exercise capacity in adolescents and adults with Fontan circulation. Clin. Res. Cardiol. 103, 641–646 (2014).
Fredriksen, P. M. et al. Aerobic capacity in adults with various congenital heart diseases. Am. J. Cardiol. 87, 310–314 (2001).
Bradley, E. A., Berman, D. & Daniels, C. J. First implantable hemodynamic monitoring device placement in single ventricle fontan anatomy. Catheter. Cardiovasc. Interv. 88, 248–252 (2016).
Talwar, S. et al. Outcomes of patients undergoing primary fontan operation beyond first decade of life. World J. Pediatr. Congenit. Heart Surg. 8, 487–494 (2017).
Ohuchi, H. Cardiopulmonary response to exercise in patients with the Fontan circulation. Cardiol. Young-. 15(Suppl 3), 39–44 (2005).
Khiabani, R. H. et al. Exercise capacity in single-ventricle patients after Fontan correlates with haemodynamic energy loss in TCPC. Heart 101, 139–143 (2015).
Fu, Q., Colgan, S. P. & Shelley, C. S. Hypoxia: the force that drives chronic kidney disease. Clin. Med. Res. 14, 15–39 (2016).
Opotowsky, A. R. et al. Estimated glomerular filtration rate and urine biomarkers in patients with single-ventricle Fontan circulation. Heart 103, 434–442 (2016).
Acknowledgements
This work was supported by the German Heart Foundation and German Society of Pediatric Cardiology (DGPK) through the Gerd-Killian grant. The presented work was performed in fulfillment of the requirements for obtaining the degree “Dr. med” at “Friedrich-Alexander University of Erlangen-Nürnberg (FAU)” of Simon Schröer. This work was performed in close cooperation with the German competence network for congenital heart disease. We thank our biostatistician D. Keller (Statistics and Consultation, Kürnach, Germany) for compiling the statistical analysis of the data and V. Laternser and J. Halbfass for English language editing. The paper was presented as a poster at the Annual Meeting of the Association for European Pediatric and Congenital Cardiology AEPC in Rome 2016.
Author information
Authors and Affiliations
Contributions
J.M. and O.T. designed the study and contributed to the paper. S.D., R.C., H.R.T. and F.B.F. contributed to the design of the study. F.M. and A.R. offered the technical expertise of NIRS measurements. J.M., M.A., S.S. and F.M. collected the data. S.S., F.M. and J.M. analyzed and interpreted the data and drafted the paper. M.R. carried out the laboratory analysis of the data. All participating authors critically revised the paper before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Schröer, S., Fahlbusch, F.B., Münch, F. et al. Multisite measurement of regional oxygen saturation in Fontan patients with and without protein-losing enteropathy at rest and during exercise. Pediatr Res 85, 777–785 (2019). https://doi.org/10.1038/s41390-019-0346-3
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-019-0346-3