Abstract
Background
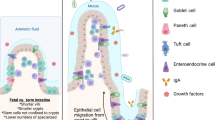
Preterm infants are susceptible to unique pathology due to their immaturity. Mouse models are commonly used to study immature intestinal disease, including necrotizing enterocolitis (NEC). Current NEC models are performed at a variety of ages, but data directly comparing intestinal developmental stage equivalency between mice and humans are lacking.
Methods
Small intestines were harvested from C57BL/6 mice at 3–4 days intervals from birth to P28 (n = 8 at each age). Preterm human small intestine samples representing 17–23 weeks of completed gestation were obtained from the University of Pittsburgh Health Sciences Tissue Bank, and at term gestation during reanastamoses after resection for NEC (n = 4–7 at each age). Quantification of intestinal epithelial cell types and messenger RNA for marker genes were evaluated on both species.
Results
Overall, murine and human developmental trends over time are markedly similar. Murine intestine prior to P10 is most similar to human fetal intestine prior to viability. Murine intestine at P14 is most similar to human intestine at 22–23 weeks completed gestation, and P28 murine intestine is most similar to human term intestine.
Conclusion
Use of C57BL/6J mice to model the human immature intestine is reasonable, but the age of mouse chosen is a critical factor in model development.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Howson, C. P., Kinney, M. V., McDougall, L., Lawn, J. E., Born Too Soon Preterm Birth Action Group. Born too soon: preterm birth matters. Reprod. Health 10 (Suppl. 1), S1 (2013).
Patel, R. M. et al. Causes and timing of death in extremely premature infants from 2000 through 2011. N. Engl. J. Med. 372, 331–340 (2015).
Walsh, M. C. et al. Neonatal outcomes of moderately preterm infants compared to extremely preterm infants. Pediatr. Res. 82, 297–304 (2017).
Ares, G. J., McElroy, S. J. & Hunter, C. J. The science and necessity of using animal models in the study of necrotizing enterocolitis. Semin. Pediatr. Surg. 27, 29–33 (2018).
Jilling, T. et al. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J. Immunol. 177, 3273–3282 (2006).
Halpern, M. D. et al. Decreased development of necrotizing enterocolitis in IL-18-deficient mice. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G20–G26 (2008).
Good, M. et al. The human milk oligosaccharide 2′-fucosyllactose attenuates the severity of experimental necrotising enterocolitis by enhancing mesenteric perfusion in the neonatal intestine. Br. J. Nutr. 116, 1175–1187 (2016).
MohanKumar, K. et al. Gut mucosal injury in neonates is marked by macrophage infiltration in contrast to pleomorphic infiltrates in adult: evidence from an animal model. Am. J. Physiol. Gastrointest. Liver Physiol. 303, G93–G102 (2012).
White, J. R., Gong, H., Pope, B., Schlievert, P. & McElroy, S. J. Paneth-cell-disruption-induced necrotizing enterocolitis in mice requires live bacteria and occurs independently of TLR4 signaling. Dis. Model. Mech. 10, 727–736 (2017).
McElroy, S. J. & Weitkamp, J. H. Innate immunity in the small intestine of the preterm infant. NeoReviews 12, e517–e526 (2011).
Nguyen, T. L., Vieira-Silva, S., Liston, A. & Raes, J. How informative is the mouse for human gut microbiota research? Dis. Model. Mech. 8, 1–16 (2015).
Hugenholtz, F. & de Vos, W. M. Mouse models for human intestinal microbiota research: a critical evaluation. Cell. Mol. Life Sci. 75, 149–160 (2018).
Mestas, J. & Hughes, C. C. Of mice and not men: differences between mouse and human immunology. J. Immunol. 172, 2731–2738 (2004).
Yee, W. H. et al. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics 129, e298–e304 (2012).
McElroy, S. J. et al. The ErbB4 ligand neuregulin-4 protects against experimental necrotizing enterocolitis. Am. J. Pathol. 184, 2768–2778 (2014).
Fricke, E. M. et al. Lipopolysaccharide-induced maternal inflammation induces direct placental injury without alteration in placental blood flow and induces a secondary fetal intestinal injury that persists into adulthood. Am. J. Reprod. Immunol. 79, e12816 (2018).
Good, M. et al. Breast milk protects against the development of necrotizing enterocolitis through inhibition of Toll-like receptor 4 in the intestinal epithelium via activation of the epidermal growth factor receptor. Mucosal Immunol. 8, 1166–1179 (2015).
Helander, H. F. & Fandriks, L. Surface area of the digestive tract—revisited. Scand. J. Gastroenterol. 49, 681–689 (2014).
Gilbert, J. A. et al. Current understanding of the human microbiome. Nat. Med. 24, 392–400 (2018).
Patel, R. M., Rysavy, M. A., Bell, E. F. & Tyson, J. E. Survival of infants born at periviable gestational ages. Clin. Perinatol. 44, 287–303 (2017).
Younge, N. et al. Survival and neurodevelopmental outcomes among periviable infants. N. Engl. J. Med. 376, 617–628 (2017).
Sangild, P. T. et al. Diet- and colonization-dependent intestinal dysfunction predisposes to necrotizing enterocolitis in preterm pigs. Gastroenterology 130, 1776–1792 (2006).
Waligora-Dupriet, A. J., Dugay, A., Auzeil, N., Huerre, M. & Butel, M. J. Evidence for clostridial implication in necrotizing enterocolitis through bacterial fermentation in a gnotobiotic quail model. Pediatr. Res. 58, 629–635 (2005).
Gonzalez, L. M., Moeser, A. J. & Blikslager, A. T. Porcine models of digestive disease: the future of large animal translational research. Transl. Res. 166, 12–27 (2015).
Myer, M. S. Paneth cells in the pig-a controversial issue. J. S Afr. Vet. Assoc. 53, 69 (1982).
Puiman, P. J., Stoll, B., van Goudoever, J. B. & Burrin, D. G. Enteral arginine does not increase superior mesenteric arterial blood flow but induces mucosal growth in neonatal pigs. J. Nutr. 141, 63–70 (2011).
Ziegler, A., Gonzalez, L. & Blikslager, A. Large animal models: the key to translational discovery in digestive disease research. Cell. Mol. Gastroenterol. Hepatol. 2, 716–724 (2016).
Litten-Brown, J. C., Corson, A. M. & Clarke, L. Porcine models for the metabolic syndrome, digestive and bone disorders: a general overview. Animal 4, 899–920 (2010).
McCracken, V. J. & Lorenz, R. G. The gastrointestinal ecosystem: a precarious alliance among epithelium, immunity and microbiota. Cell Microbiol. 3, 1–11 (2001).
van der Flier, L. G. & Clevers, H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 71, 241–260 (2009).
Cheng, H. & Leblond, C. P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am. J. Anat. 141, 537–561 (1974).
Yan, K. S. et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc. Natl. Acad. Sci. USA 109, 466–471 (2012).
Frey, M. R. & Brent Polk, D. ErbB receptors and their growth factor ligands in pediatric intestinal inflammation. Pediatr. Res. 75, 127–132 (2014).
Almohazey, D. et al. The ErbB3 receptor tyrosine kinase negatively regulates Paneth cells by PI3K-dependent suppression of Atoh1. Cell Death Differ. 24, 855–865 (2017).
Kandasamy, J., Huda, S., Ambalavanan, N. & Jilling, T. Inflammatory signals that regulate intestinal epithelial renewal, differentiation, migration and cell death: implications for necrotizing enterocolitis. Pathophysiology 21, 67–80 (2014).
Clevers, H. C. & Bevins, C. L. Paneth cells: maestros of the small intestinal crypts. Annu. Rev. Physiol. 75, 289–311 (2013).
Berman, L. & Moss, R. L. Necrotizing enterocolitis: an update. Semin. Fetal Neonatal Med. 16, 145–150 (2011).
Bevins, C. L. & Salzman, N. H. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol. 9, 356–368 (2011).
Ralls, M. W., Gadepalli, S. K., Sylvester, K. G. & Good, M. Development of the necrotizing enterocolitis society registry and biorepository. Semin. Pediatr. Surg. 27, 25–28 (2018).
Dowling, R. H. & Booth, C. C. Functional compensation after small-bowel resection in man. Demonstration by direct measurement. Lancet 2, 146–147 (1966).
Acknowledgements
S.J.M. is supported by the National Institutes of Health DK097335 and the Stead Family Department of Pediatrics, Carver College of Medicine at the University of Iowa. M.G. is supported by K08DK101608, R03DK111473, and R01DK118568 from the National Institutes of Health, March of Dimes Foundation Grant No. 5-FY17-79, the Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital, and the Department of Pediatrics at Washington University School of Medicine, St. Louis. M.R.F. is supported by R01DK095004 and a Senior Research Award from the Crohn’s and Colitis Foundation.
Author information
Authors and Affiliations
Contributions
A.H.S., S.R.L., M.R.F., M.G., and S.J.M. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. M.G. and S.J.M. are co-senior/corresponding authors. All authors reviewed the results and approved the final version of the manuscript. Concept and design: A.H.S., M.R.F., M.G., and S.J.M. Acquisition, analysis, and interpretation of data: A.H.S., H.G., M.N., A.N.L., Q.G., W.E.L., J.J.H., S.R.L., M.R.F., M.G., and S.J.M. conducted all experimental bench work and contributed to sample analyses. Q.G. and M.G. maintained the clinical database. Statistical analysis: A.H.S., A.N.L., Q.G., W.E.L., S.R.L., M.G., and S.J.M. Manuscript preparation, drafting, and critical revisions: A.H.S., H.G., M.N., A.N.L., Q.G., W.E.L., J.J.H., S.R.L., M.R.F., M.G., and S.J.M. prepared, drafted, and critically revised the manuscript. Study supervision: M.R.F., M.G., and S.J.M.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Stanford, A.H., Gong, H., Noonan, M. et al. A direct comparison of mouse and human intestinal development using epithelial gene expression patterns. Pediatr Res 88, 66–76 (2020). https://doi.org/10.1038/s41390-019-0472-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-019-0472-y
This article is cited by
-
Thrombin-preconditioned mesenchymal stromal cell-derived extracellular vesicles attenuate experimental necrotizing enterocolitis
Stem Cell Research & Therapy (2025)
-
Temporal mapping of epithelial vs. immune cell inflammatory shifts in the neonatal intestinal mucosa
Pediatric Research (2025)
-
Murine intestinal epithelial cells and lymphocytes undergo contrasting inflammatory shifts during gastrointestinal development
Pediatric Research (2025)
-
Single-cell map of dynamic cellular microenvironment of radiation-induced intestinal injury
Communications Biology (2023)
-
Intestinal epithelium in early life
Mucosal Immunology (2022)