Abstract
Background
Decreased expression of the renal aquaporin (AQP) protein family is associated with hydronephrosis in adult humans and animals. However, the expression of AQPs, especially subtypes AQP1–3, which play a core role in the urinary concentration function, in hydronephrotic human fetuses is not clear. The aim of this study is to investigate the expression of the AQP1–3 in normal and hydronephrotic human fetal kidneys.
Methods
Twenty-one normal and six hydronephrotic kidney (HK) samples were harvested from abortive fetuses. Meanwhile, seven normal adult human kidney samples were collected as positive controls. Quantitative real-time PCR, western blotting, and immunohistochemistry were used to analyze the expression of AQP1–3.
Results
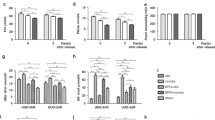
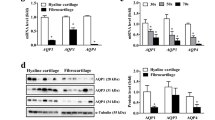
Both the protein and messenger mRNA expression levels of AQP1–3 increased with gestational age in the normal fetuses, but the levels were significantly lower than those in the adult tissues and significantly higher than those in the hydronephrotic fetuses at the same gestational age.
Conclusions
The increased expression of AQP1–3 with gestational age in the fetal kidney may indicate maturation of the urinary concentrating ability. The lower expression of AQP1–3 in HKs may reflect a maturation obstacle with regard to urinary concentration in human hydronephrotic fetuses.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Shimada, K. et al. Urological emergency in neonates with congential hydronephrosis. Int. J. Urol. 14, 388–392 (2007).
Chevalier, R. L. Molecular and cellular pathophysiology of obstructive nephropathy. Pediatr. Nephrol. 13, 612–619 (1999).
McDill, BradleyW. et al. Congenital progressive hydronephrosis (cph) is caused by an S256L mutation in aquaporin-2 that affects its phosphorylation and apical membrane accumulation. Proc. Natl. Acad. Sci. USA 103, 6952–6957 (2006).
Ardissino, G. et al. Epidemiology of chronic renal failure in children: data from the ItalKid project. Pediatrics 111, e382–e387 (2003).
Wen, J. G. et al. Expression of renal aquaporins is down-regulated in children with congenital hydronephrosis. Scand. J. Urol. Nephrol. 43, 486–493 (2009).
Li, Z. Z. et al. Decrease of renal aquaporins 1–4 is associated with renal function impairment in pediatric congenital hydronephrosis. World J. Pediatr. 8, 335–341 (2012).
Parreira, K. S. et al. Expression patterns of the aquaporin gene family during renal development: influence of genetic variability. Pflug. Arch. 458, 745–759 (2009).
Zelenina, M., Zelenin, S. & Aperia, A. Water channels (aquaporins) and their role for postnatal adaptation. Pediatr. Res. 57, 47R–53R (2005).
Nielsen, S. et al. Aquaporins kidney: from molecules to medicine. Mol. Med. Physiol. Rev. 82, 205–244 (2002).
Schnermann, J. et al. Defective proximal tubular fluid reabsorption in transgenic aquaporin-1 null mice. Proc. Natl. Acad. Sci. USA 95, 9660–9664 (1998).
Chou, C. L. et al. Reduced water permeability and altered ultrastructure in thin descending limb of Henle in aquaporin-1 null mice. J. Clin. Invest. 103, 491–496 (1999).
Ma, T. et al. Severely impaired urinary concentrating ability in transgenic mice lacking aquaporin-1 water channels. J. Biol. Chem. 273, 4296–4299 (1998).
Kwon, T. H. et al. Aquaporins in the kidney. Handb. Exp. Pharmacol. 190, 95–132 (2009).
Liu, H. et al. Aquaporin gene expression and regulation in the ovine fetal lung. J. Physiol. 551, 503–514 (2003).
Robben, J. H. & Knoers, N. V. Deen PM.Cell biological aspects of the vasopressin type-2 receptor and aquaporin 2 water channel in nephrogenic diabetes insipidus. Am. J. Physiol. Ren. Physiol. 291, F257–F270 (2006).
Boccalandro, C. et al. Characterization of an aquaporin-2 water channel gene mutation causing partial nephrogenic diabetes insipidus in a Mexican family: evidence of increased frequency of the mutation in the town of origin. J. Am. Soc. Nephrol. 15, 1223–1231 (2004).
Frigeri, A. et al. Immunolocalization of the mercurial-insensitive water channel and glycerol intrinsic protein in epithelial cell plasma membranes. Proc. Natl. Acad. Sci. USA 92, 4328–4331 (1995).
Ecelbarger, C. A. et al. Aquaporin-3 water channel localization and regulation in rat kidney. Am. J. Physiol. 269, F663–F672 (1995).
Ma, T. et al. Nephrogenic diabetes insipidus in mice lacking aquaporin-3 water channels. Proc. Natl. Acad. Sci. USA 97, 4386–4391 (2000).
Lindower, J. B. Water balance in the fetus and neonate. Semin. Fetal Neonatal Med. 22, 71–75 (2017).
Xing, L. et al. Ontogeny of the mammalian kidney: expression of aquaporins 1, 2, 3, and 4. World J. Pediatr. 10, 306–312 (2014).
Nguyen, H. T. et al. The Society for Fetal Urology consensus statement on the evaluation and management of antenatal hydronephrosis. J. Pediatr. Urol. 6, 212–231 (2010).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 25, 402–408 (2001).
Yamamoto, T. et al. Expression of AQP family in rat kidneys during development and maturation. Am. J. Physiol. 272, F198–F204 (1997).
Baum, M. A. et al. The perinatal expression of aquaporin-2 and aquaporin-3 in developing kidney. Pediatr. Res. 43, 783–790 (1998).
Smith, B. L. et al. Concurrent expression of erythroid and renal aquaporin CHIP and appearance of water channel activity in perinatal rats. J. Clin. Invest. 92, 2035–2041 (1993).
Wintour, E. M. et al. Ovine AQP1: cDNA cloning, ontogeny, and control of renal gene expression. Pediatr. Nephrol. 12, 545–553 (1998).
Butkus, A. et al. Ovine aquaporin-2: cDNA cloning, ontogeny and control of renal gene expression. Pedia. Nephrol. 13, 379–390 (1999).
Xing, L. & Nørregaard, R. Influence of sex on aquaporin1-4 and vasopressin V2 receptor expression in the pig kidney during development. Pediatr. Res. 80, 452–459 (2016).
Chevalier, R. L. Chronic partial ureteral obstruction and the developing kidney. Pediatr. Radiol. 38, S35–S40 (2008).
Manucha, W. Biochemical molecular markers in unilateral ureteral obstruction. Biocell 31, 1–12 (2007).
Frøkiaer, J. et al. Renal aquaporins and sodium transporters with special focus on urinary tract obstruction. APMIS Suppl. 109, 71–79 (2003).
Shi, Y. et al. Neonatal ureteral obstruction alters expressionof renal sodium transporters and aquaporin water channels. Kidney Int. 66, 203–215 (2004).
Nørregaard, R. et al. COX-2 activity transiently contributes to increased water and NaCl excretion in the polyuric phase after release of ureteral obstruction. Am. J. Physiol. Ren. Physiol. 292, F1322–F1333 (2007).
Lindower, J. B. Water balance in the fetus and neonate. Semin. Fetal Neonatal Med. 22, 71–75 (2017).
Acknowledgements
We would like to thank Kai Yang, who was the head nurse in the delivery room, and Xianlan Zhao, chief of the Department of Obstetrics, for helping us to collect the fetal kidney samples. We also would like to thank Xiaoping Shang, the statistician of the Department of Medical Record Room, for the statistical analysis help. This study was funded by the National Science Foundation of China (81370869 and 81370689).
Author information
Authors and Affiliations
Contributions
J.W., J.F., S.Y., Y.C., L.H., L.W., X.G., Y.W., Y.L., X.H., Z.H., C.R., Z.J., Z.G., R.Z., J.W., and J.W. made substantial contributions to the conception and design of the study, acquisition of the data, or analysis and interpretation of the data; J.F., J.W., S.Y., Y.C., L.H., L.W., X.G., and Y.W. drafted the article or revised it critically for important intellectual content; J.F., L.H., and J.W. revised the manuscript. J.W. provided final approval of the version to be published. J.F., S.Y., and Y.C. made equal contributions to the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All procedures in this study were performed in accordance with the ethical standards of the institutional research committee and in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Feng, J., Yan, S., Chen, Y. et al. Aquaporin1–3 expression in normal and hydronephrotic kidneys in the human fetus. Pediatr Res 86, 595–602 (2019). https://doi.org/10.1038/s41390-019-0485-6
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-019-0485-6
This article is cited by
-
Erythropoietin prevented the decreased expression of aquaporin1–3 in ureteral obstructive kidneys in juvenile rats
Pediatric Research (2023)
-
Alteration of N6-methyladenosine epitranscriptome profiles in bilateral ureteral obstruction-induced obstructive nephropathy in juvenile rats
Pediatric Research (2023)