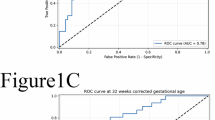
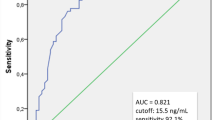
Abstract
Background
Human chorionic gonadotropin (hCG) and luteinizing hormone (LH) are pro-angiogenic gonadotropic hormones, which classically target the reproductive organs. However, hCG, LH, and their shared CG/LH receptor are also present in the human eye. The possibility that a deficiency of these hormones may be involved in the pathogenesis of retinopathy of prematurity (ROP) during its early non-proliferative phase has not been explored.
Methods
We conducted a cross-sectional study of Michigan-born preterm infants utilizing dried blood spots. We analyzed hCG and LH blood levels at 1 week and 4 weeks of age from 113 study participants (60 without ROP; 53 with non-proliferative ROP). We utilized electrochemiluminescence assays on the Mesoscale Discovery platform.
Results
Similar levels of hCG are found in preterm infants at both 1 week and 4 weeks after birth. Preterm infants with non-proliferative ROP, after adjusting for sex and gestational age, have 2.42 [95% CI: 1.08–5.40] times the odds of having low hCG at fourth week of age.
Conclusions
We found that hCG is present postnatally in preterm infants and that a deficiency of hCG at 4 weeks of age is potentially associated with non-proliferative ROP. This provides novel evidence to suggest that hCG may participate in human retinal angiogenesis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ferrara, N. Vascular endothelial growth factor: basic science and clinical progress. Endocr. Rev. 25, 581–611 (2004).
Gogat, K. et al. VEGF and KDR gene expression during human embryonic and fetal eye development. Invest. Ophthalmol. Vis. Sci. 45, 7–14 (2004).
Ma, I. T. et al. VEGF mRNA and protein concentrations in the developing human eye. Pediatr. Res. 77, 500–505 (2015).
Chan-Ling, T., Gole, G. A., Quinn, G. E., Adamson, S. J. & Darlow, B. A. Pathophysiology, screening and treatment of ROP: a multi-disciplinary perspective. Prog. Retin. Eye Res. 62, 77–119 (2018).
Hartnett, M. E. Pathophysiology and mechanisms of severe retinopathy of prematurity. Ophthalmology 122, 200–210 (2015).
Hartnett, M. E. & Penn, J. S. Mechanisms and management of retinopathy of prematurity. N. Engl. J. Med. 368, 1162–1163 (2013).
Hellstrom, A., Smith, L. E. & Dammann, O. Retinopathy of prematurity. Lancet 382, 1445–1457 (2013).
Stone, J. et al. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J. Neurosci. 15, 4738–4747 (1995).
Baba, T. et al. VEGF165b in the developing vasculatures of the fetal human eye. Dev. Dyn. 241, 595–607 (2012).
Slomiany, M. G. & Rosenzweig, S. A. Hypoxia-inducible factor-1-dependent and -independent regulation of insulin-like growth factor-1-stimulated vascular endothelial growth factor secretion. J. Pharm. Exp. Ther. 318, 666–675 (2006).
Cole, L. A. Biological functions of hCG and hCG-related molecules. Reprod. Biol. Endocrinol. 8, 102 (2010).
Nwabuobi, C. et al. hCG: biological functions and clinical applications. Int. J. Mol. Sci. 18, E2037 (2017).
Trau, H. A., Davis, J. S. & Duffy, D. M. Angiogenesis in the primate ovulatory follicle is stimulated by luteinizing hormone via prostaglandin E2. Biol. Reprod. 92, 15 (2015).
Tsampalas, M. et al. Human chorionic gonadotropin: a hormone with immunological and angiogenic properties. J. Reprod. Immunol. 85, 93–98 (2010).
Dukic-Stefanovic, S. et al. Chorionic gonadotropin and its receptor are both expressed in human retina, possible implications in normal and pathological conditions. PLoS ONE 7, e52567 (2012).
Movsas, T. Z. et al. Confirmation of luteinizing hormone (LH) in living human vitreous and the effect of LH receptor reduction on murine electroretinogram. Neuroscience 385, 1–10 (2018).
Movsas, T. Z., Sigler, R. & Muthusamy, A. Elimination of signaling by the luteinizing hormone receptor reduces ocular VEGF and retinal vascularization during mouse eye development. Curr. Eye Res. 43, 1–4 (2018).
Movsas, T. Z., Sigler, R. & Muthusamy, A. Vitreous levels of luteinizing hormone and VEGF are strongly correlated in healthy mammalian eyes. Curr. Eye Res. 43, 1041–1044 (2018).
Movsas, T. Z. & Muthusamy, A. The potential effect of human chorionic gonadotropin on vasoproliferative disorders of the immature retina. Neuroreport 29, 1525–1529 (2018).
Huhtaniemi, I. T., Korenbrot, C. C. & Jaffe, R. B. Content of chorionic gonadotropin in human fetal tissues. J. Clin. Endocrinol. Metab. 46, 994–997 (1978).
Melmed, S., Polonsky, K., S., Reed Larsen, P. & Kroneberg, H. Williams Textbook of Endocrinology 12th edn (Elsevier, Philadelphia, PA, 2011).
Jauniaux, E., Pahal, G., Gervy, C. & Gulbis, B. Blood biochemistry and endocrinology in the human fetus between 11 and 17 weeks of gestation. Reprod. Biomed. Online 1, 38–44 (2000).
Browne, J. L., Schielen, P. C., Belmouden, I., Pennings, J. L. & Klipstein-Grobusch, K. Dried blood spot measurement of pregnancy-associated plasma protein A (PAPP-A) and free beta-subunit of human chorionic gonadotropin (beta-hCG) from a low-resource setting. Prenat. Diagn. 35, 592–597 (2015).
Edelman, A., Stouffer, R., Zava, D. T. & Jensen, J. T. A comparison of blood spot vs. plasma analysis of gonadotropin and ovarian steroid hormone levels in reproductive-age women. Fertil. Steril. 88, 1404–1407 (2007).
Gruner, N., Stambouli, O. & Ross, R. S. Dried blood spots—preparing and processing for use in immunoassays and in molecular techniques. J. Vis. Exp. 52619 (2015).
Chaturvedi, S., Dell, E., Siegel, D., Brittingham, G. & Seetharam, S. Development of a rapid streptavidin capture-based assay for the tyrosine phosphorylated CSF-1R in peripheral blood mononuclear cells. Int. J. Biol. Sci. 9, 1099–1107 (2013).
Zhao, X. et al. A clinical biomarker assay to quantitate thymic stromal lymphopoietin in human plasma at sub-pg/ml level. Bioanalysis 7, 573–582 (2015).
Leviton, A. et al. Antecedents and early correlates of high and low concentrations of angiogenic proteins in extremely preterm newborns. Clin. Chim. Acta 471, 1–5 (2017).
Ellington, A. A., Kullo, I. J., Bailey, K. R. & Klee, G. G. Antibody-based protein multiplex platforms: technical and operational challenges. Clin. Chem. 56, 186–193 (2010).
Liew, M. et al. Validating a custom multiplex ELISA against individual commercial immunoassays using clinical samples. Biotechniques 42, 327–328 (2007). 30-3.
Toedter, G., Hayden, K., Wagner, C. & Brodmerkel, C. Simultaneous detection of eight analytes in human serum by two commercially available platforms for multiplex cytokine analysis. Clin. Vaccin. Immunol. 15, 42–48 (2008).
McGregor, W. G., Raymoure, W. J., Kuhn, R. W. & Jaffe, R. B. Fetal tissue can synthesize a placental hormone. Evidence for chorionic gonadotropin beta-subunit synthesis by human fetal kidney. J. Clin. Invest. 68, 306–309 (1981).
Lei, Z. M. et al. Targeted disruption of luteinizing hormone/human chorionic gonadotropin receptor gene. Mol. Endocrinol. 15, 184–200 (2001).
Greaves, R. F., Hunt, R. W., Chiriano, A. S. & Zacharin, M. R. Luteinizing hormone and follicle-stimulating hormone levels in extreme prematurity: development of reference intervals. Pediatrics 121, e574–e580 (2008).
Egeland, S. V., Reubsaet, L., Paus, E. & Halvorsen, T. G. Dual-immuno-MS technique for improved differentiation power in heterodimeric protein biomarker analysis: determination and differentiation of human chorionic gonadotropin variants in serum. Anal. Bioanal. Chem. 408, 7379–7391 (2016).
Wagner, M., Tonoli, D., Varesio, E. & Hopfgartner, G. The use of mass spectrometry to analyze dried blood spots. Mass Spectrom. Rev. 35, 361–438 (2016).
Fichorova, R. N. et al. Biological and technical variables affecting immunoassay recovery of cytokines from human serum and simulated vaginal fluid: a multicenter study. Anal. Chem. 80, 4741–4751 (2008).
Acknowledgements
We would like to acknowledge the Michigan Neonatal Biobank, who provided the carefully preserved neonatal dried blood spots. In addition, we would like to acknowledge the staff of the Michigan Newborn Screening Program, the Michigan BioTrust for Health program, and the state registrar at the Michigan Department of Health and Human Services. Their expert skill in performing the complex database matching procedure was critical to this study’s success. This study was funded by NIH/NEI grant #R43EY028467.
Author information
Authors and Affiliations
Contributions
Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data and final approval of the version to be published: All the authors. Drafting the article or revising it critically for important intellectual content: T.Z.M., N.P., I.H.G., Q.L., A.M.
Corresponding author
Ethics declarations
Competing interests
T.Z.M. is the Director and Principal Investigator at Zietchick Research Institute (ZRI), a private (for-profit) research institute, and has pending patent applications for the use of both gonadotropins and gonadotropin antagonists for the treatment of ocular disorders. A.M. is a salaried employee (no equity) at ZRI. The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Movsas, T.Z., Paneth, N., Gewolb, I.H. et al. The postnatal presence of human chorionic gonadotropin in preterm infants and its potential inverse association with retinopathy of prematurity. Pediatr Res 87, 558–563 (2020). https://doi.org/10.1038/s41390-019-0580-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-019-0580-8