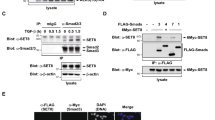
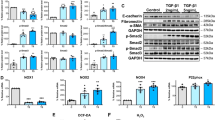
Abstract
Backgrounds
To investigate the potential mechanism of hypospadias induced by DEHP in rats to reveal the preventative effect of TGF-β1 in hypospadias induced by DEHP via the reduction of EMT.
Methods
Time-mated Sprague-Dawley rats underwent cesarean section, and the penises of male pups were collected after exposure to corn oil or DEHP to establish a rat model of hypospadias and to further study the molecular mechanisms of hypospadias in vivo. In addition, the penises were cultured and treated with MEHP or MEHP+TGF-β1 in vitro. Subsequently, histomorphology and elements in TGF-β/Smad signaling pathway changes were evaluated using scanning electron microscopy, immunofluorescence, polymerase chain reaction, and western blot.
Results
The development of rat penis and urethral seam fusion were delayed after the treatment with DEHP in vivo or MEHP in vitro compared with the Control group. Moreover, TGF-β1, Smad2/Smad3, and the mesenchymal biomarkers, including α-SMA, N-cadherin, and Vimentin, were decreased. However, the epithelial biomarkers, including E-cadherin, ZO-1, β-catenin, and occludin, were increased. In addition, TGF-β1 could relieve all of the above changes.
Conclusion
Gestational DEHP exposure could lead to hypospadias by reducing urethral EMT. Moreover, TGF-β1 could prevent it by regenerating EMT through activating the TGF-β/Smad signal pathway.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Rübben, I. & Stein, R. [Hypospadias: insights and challenges]. Urol. A 56, 1256–1265 (2017).
Bouty, A. et al. The genetic and environmental factors underlying hypospadias. Sex. Dev. 9, 239–259 (2015).
Carmichael, S. L., Shaw, G. M. & Lammer, E. J. Environmental and genetic contributors to hypospadias: a review of the epidemiologic evidence. Birth Defects Res. A Clin. Mol. Teratol. 94, 499–510 (2012).
Craig, J. R. et al. Management of adults with prior failed hypospadias surgery. Transl. Androl. Urol. 3, 196–204 (2014).
Unüvar, T. & Büyükgebiz, A. Fetal and neonatal endocrine disruptors. J. Clin. Res. Pediatr. Endocrinol. 4, 51–60 (2012).
Hu, G. X. et al. Phthalate-induced testicular dysgenesis syndrome: Leydig cell influence. Trends Endocrinol. Metab. 20, 139–145 (2009).
Santangeli, S. et al. Effects of diisononyl phthalate on Danio rerio reproduction. Environ. Pollut. 231, 1051–1062 (2017).
Liu, L. et al. Phthalate metabolites related to infertile biomarkers and infertility in Chinese men. Environ. Pollut. 231, 291–300 (2017).
Halden, R. U. Plastics and health risks. Annu. Rev. Public Health 31, 179–194 (2010).
Heudorf, U., Mersch-Sundermann, V. & Angerer, J. Phthalates: toxicology and exposure. Int. J. Hyg. Environ. Health 210, 623–634 (2007).
Swan, S. H. et al. First trimester phthalate exposure and anogenital distance in newborns. Hum. Reprod. 30, 963–972 (2015).
Li, M. et al. Dose-related effect by maternal exposure to di-(2-ethylhexyl) phthalate plasticizer on inducing hypospadiac male rats. Environ. Toxicol. Pharmacol. 35, 55–60 (2013).
Posnack, N. G. et al. Gene expression profiling of DEHP-treated cardiomyocytes reveals potential causes of phthalate arrhythmogenicity. Toxicology 279, 54–64 (2011).
Kamstra, J. H. et al. Differential DNA methylation at conserved non-genic elements and evidence for transgenerational inheritance following developmental exposure to mono(2-ethylhexyl) phthalate and 5-azacytidine in zebrafish. Epigenetics Chromatin 10, 20 (2017).
Deutschle, T. et al. A controlled challenge study on di(2-ethylhexyl) phthalate (DEHP) in house dust and the immune response in human nasal mucosa of allergic subjects. Environ. Health Perspect. 116, 1487–1493 (2008).
Connolly, E. C., Freimuth, J. & Akhurst, R. J. Complexities of TGF-β targeted cancer therapy. Int. J. Biol. Sci. 8, 964–978 (2012).
Kubiczkova, L. et al. TGF-β - an excellent servant but a bad master. J. Transl. Med. 10, 183 (2012).
Larue, L. & Bellacosa, A. Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3’ kinase/AKT pathways. Oncogene 24, 7443–7454 (2005).
Thompson, E. W., Newgreen, D. F. & Tarin, D. Carcinoma invasion and metastasis: a role for epithelial-mesenchymal transition. Cancer Res. 65, 5991–5995 (2005). Discussion 5995.
Zhou, Y. et al. Epithelial-mesenchymal transformation and apoptosis in rat urethra development. Pediatr. Res. 82, 1073–1079 (2017).
Baskin, L. S. et al. Urethral seam formation and hypospadias. Cell Tissue Res. 305, 379–387 (2001).
Shen, M. et al. Transforming growth factor β1 signaling coincides with epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation in the development of adenomyosis in mice. Hum. Reprod. 31, 355–369 (2016).
Morgan, E. A. et al. Loss of Bmp7 and Fgf8 signaling in Hoxa13-mutant mice causes hypospadia. Development 130, 3095–3109 (2003).
Koo, H. J. & Lee, B. M. Estimated exposure to phthalates in cosmetics and risk assessment. J. Toxicol. Environ. Health A 67, 1901–1914 (2004).
Huber, W. W., Grasl-Kraupp, B. & Schulte-Hermann, R. Hepatocarcinogenic potential of di(2-ethylhexyl)phthalate in rodents and its implications on human risk. Crit. Rev. Toxicol. 26, 365–481 (1996).
Barlow, N. J. & Foster, P. M. Pathogenesis of male reproductive tract lesions from gestation through adulthood following in utero exposure to Di(n-butyl) phthalate. Toxicol. Pathol. 31, 397–410 (2003).
Do, R. P. et al. Non-monotonic dose effects of in utero exposure to di(2-ethylhexyl) phthalate (DEHP) on testicular and serum testosterone and anogenital distance in male mouse fetuses. Reprod. Toxicol. 34, 614–621 (2012).
Koch, H. M., Preuss, R. & Angerer, J. Di(2-ethylhexyl)phthalate (DEHP): human metabolism and internal exposure–an update and latest results. Int. J. Androl. 29, 155–165 (2006). Discussion 181–185.
Mahood, I. K. et al. Abnormal Leydig Cell aggregation in the fetal testis of rats exposed to di (n-butyl) phthalate and its possible role in testicular dysgenesis. Endocrinology 146, 613–623 (2005).
Haraguchi, R. et al. Unique functions of Sonic hedgehog signaling during external genitalia development. Development 128, 4241–4250 (2001).
Zhang, J., Tian, X. J. & Xing, J. Signal transduction pathways of EMT induced by TGF-β, SHH, and WNT and their crosstalks. J. Clin. Med. 5, E41 (2016).
Liu, X. et al. Di(2-ethylhexyl) phthalate (DEHP) increases transforming growth factor-beta1 expression in fetal mouse genital tubercles. J. Toxicol. Environ. Health A 71, 1289–1294 (2008).
Willingham, E. & Baskin, L. S. Candidate genes and their response to environmental agents in the etiology of hypospadias. Nat. Clin. Pract. Urol. 4, 270–279 (2007).
Chen, T. et al. Mutation screening of BMP4, BMP7, HOXA4 and HOXB6 genes in Chinese patients with hypospadias. Eur. J. Hum. Genet. 15, 23–28 (2007).
Baskin, L. S. et al. Cellular signaling in the bladder. Front. Biosci. 2, d592–d595 (1997).
Valcourt, U. et al. TGF-beta and the Smad signaling pathway support transcriptomic reprogramming during epithelial-mesenchymal cell transition. Mol. Biol. Cell 16, 1987–2002 (2005).
Acknowledgements
This study was supported by the National Natural Science Foundation of China (grant 81771566) and the National Natural Science Foundation of China (grant 81970571).
Author contributions
X.L. and G.W. conceived and designed the experiments; Yue Zhou performed the experiments; Yue Zhou analyzed the data; C.L. and L.S. contributed reagents/materials/analysis tools; Yue Zhou and X.L. wrote the paper. F.H., Y.L., D.L., and Yu Zhou assisted to perform the experiment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, Y., Huang, F., Liu, Y. et al. TGF-β1 relieves epithelial–mesenchymal transition reduction in hypospadias induced by DEHP in rats. Pediatr Res 87, 639–646 (2020). https://doi.org/10.1038/s41390-019-0622-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-019-0622-2
This article is cited by
-
Retrospective studies and quantitative proteomics reveal that abnormal expression of blood pressure, blood lipids, and coagulation related proteins is associated with hypospadias
Human Genetics (2024)
-
Urinary extracellular vesicles prevent di-(2-ethylhexyl) phthalate-induced hypospadias by facilitating epithelial–mesenchymal transition via PFN2 delivery
Cell Biology and Toxicology (2023)