Abstract
Background
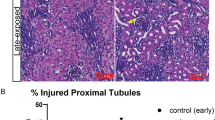
Acute kidney injury affects nearly 30% of preterm neonates in the intensive care unit. We aimed to determine whether nephrotoxin-induced AKI disrupted renal development assessed by imaging (CFE-MRI).
Methods
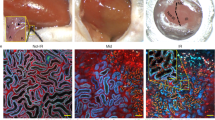
Neonatal New Zealand rabbits received indomethacin and gentamicin (AKI) or saline (control) for four days followed by cationic ferritin (CF) at six weeks. Ex vivo images were acquired using a gradient echo pulse sequence on 7 T MRI. Glomerular number (Nglom) and apparent glomerular volume (aVglom) were determined. CF toxicity was assessed at two and 28 days in healthy rabbits.
Results
Nglom was lower in the AKI group as compared to controls (74,034 vs 198,722, p < 0.01). aVglom was not different (AKI: 7.3 × 10−4 vs control: 6.2 × 10−4 mm3, p = 0.69). AKI kidneys had a band of glomeruli distributed radially in the cortex that were undetectable by MRI. Following CF injection, there was no difference in body or organ weights except for the liver, and transient changes in serum iron, platelets and white blood cell count.
Conclusions
Brief nephrotoxin exposure during nephrogenesis results in fewer glomeruli and glomerular maldevelopment in a unique pattern detectable by MRI. Whole kidney evaluation by CFE-MRI may provide an important tool to understand the development of CKD following AKI.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Hinchliffe, S. A., Sargent, P. H., Howard, C. V., Chan, Y. F. & van Velzen, D. Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab. Investig. 64, 777–784 (1991).
Rhone, E. T., Carmody, J. B., Swanson, J. R. & Charlton, J. R. Nephrotoxic medication exposure in very low birth weight infants. J. Matern. Fetal Neonatal Med. 27, 1485–1490 (2013).
Jetton, J. G. et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc. Health 1, 184–194 (2017).
Sutherland, M. R. et al. Accelerated maturation and abnormal morphology in the preterm neonatal kidney. J. Am. Soc. Nephrol. 22, 1365–1374 (2011).
Rodriguez, M. M. et al. Comparative renal histomorphometry: a case study of oligonephropathy of prematurity. Pediatr. Nephrol. 20, 945–949 (2005).
Harer, M. W., Pope, C. F., Conaway, M. R. & Charlton, J. R. Follow-up of Acute kidney injury in Neonates during Childhood Years (FANCY): a prospective cohort study. Pediatr. Nephrol. 32, 1067–1076 (2017).
Hsu, C. W., Yamamoto, K. T., Henry, R. K., De Roos, A. J. & Flynn, J. T. Prenatal risk factors for childhood CKD. J. Am. Soc. Nephrol. 25, 2105–2111 (2014).
Rule, A. D. et al. The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann. Intern. Med. 152, 561–567 (2010).
Doi, K. et al. Reduced production of creatinine limits its use as marker of kidney injury in sepsis. J. Am. Soc. Nephrol. 20, 1217–1221 (2009).
Denic, A. et al. Single-nephron glomerular filtration rate in healthy adults. N. Engl. J. Med. 376, 2349–2357 (2017).
Bertram, J. F. Estimating glomerular number: why we do it and how. Clin. Exp. Pharmacol. Physiol. 40, 785–788 (2013).
Baldelomar, E. J., Charlton, J. R., Beeman, S. C. & Bennett, K. M. Measuring rat kidney glomerular number and size in vivo with MRI. Am. J. Physiol. Ren. Physiol. 00399, 02017 (2017).
Baldelom9ar, E. J., Charlton, J. R., deRonde, K. A. & Bennett, K. M. In vivo measurement of kidney glomerular number and size in healthy and Os(/+) mice using MRI. Am. J. Physiol. Ren. Physiol. 317, F865–F873 (2019).
Baldelomar, E. J. et al. Phenotyping by magnetic resonance imaging nondestructively measures glomerular number and volume distribution in mice with and without nephron reduction. Kidney Int. 89, 498–505 (2015).
Beeman, S. C. et al. MRI-based glomerular morphology and pathology in whole human kidneys. Am. J. Physiol. Ren. Physiol. 306, F1381–F1390 (2014).
Beeman, S. C. et al. Measuring glomerular number and size in perfused kidneys using MRI. Am. J. Physiol. Ren. Physiol. 300, F1454–F1457 (2011).
Bennett, K. M. et al. MRI of the basement membrane using charged nanoparticles as contrast agents. Magn. Reson. Med. 60, 564–574 (2008).
Heilmann, M. et al. Quantification of glomerular number and size distribution in normal rat kidneys using magnetic resonance imaging. Nephrol. Dial. Transplant. 27, 100–107 (2012).
Kazimierczak, J. Histochemical study of oxidative enzymes in rabbit kidney before and after birth. Acta Anat. (Basel) 55, 352–369 (1963).
Hosaka, E. M., Santos, O. F., Seguro, A. C. & Vattimo, M. F. Effect of cyclooxygenase inhibitors on gentamicin-induced nephrotoxicity in rats. Braz. J. Med. Biol. Res. 37, 979–985 (2004).
Charlton, J. R. et al. Biocompatibility of ferritin-based nanoparticles as targeted MRI contrast agents. Nanomedicine 12, 1735–1745 (2016).
Danon, D., Goldstein, L., Marikovsky, Y. & Skutelsky, E. Use of cationized ferritin as a label of negative charges on cell surfaces. J. Ultrastruct. Res. 38, 500–510 (1972).
Moran, S. M. & Myers B. D. Course of acute renal failure studied by a model of creatinine kinetics. Kidney Int. 27, 928–937 (1985).
Gubhaju, L. et al. Is nephrogenesis affected by preterm birth? Studies in a non-human primate model. Am. J. Physiol. Ren. Physiol. 297, F1668–F1677 (2009).
Popescu, C. R. et al. Hyperoxia exposure impairs nephrogenesis in the neonatal rat: role of HIF-1alpha. PLoS ONE 8, e82421 (2013).
Kent, A. L., Brown, L., Broom, M., Broomfield, A. & Dahlstrom, J. E. Increased urinary podocytes following indomethacin suggests drug-induced glomerular injury. Pediatr. Nephrol. 27, 1111–1117 (2012).
Kent, A. L. et al. Indomethacin administered early in the postnatal period results in reduced glomerular number in the adult rat. Am. J. Physiol. Ren. Physiol. 307, F1105–F1110 (2014).
Gilbert, T., Lelievre-Pegorier, M. & Merlet-Benichou, C. Long-term effects of mild oligonephronia induced in utero by gentamicin in the rat. Pediatr. Res. 30, 450–456 (1991).
Brenner, B. M., Garcia, D. L. & Anderson, S. Glomeruli and blood pressure. Less one, more other? Am. J. Hypertens. 1, 335–347 (1988).
Brenner, B. M. & Mackenzie, H. S. Nephron mass as a risk factor for progression of renal disease. Kidney Int. Suppl. 63, S124–S127 (1997).
Kanzaki, G., et al. New insights on glomerular hyperfiltration: a Japanese autopsy study. JCI Insight. https://doi.org/10.1172/jci.insight.94334 (2017).
Keller, G., Zimmer, G., Mall, G., Ritz, E. & Amann, K. Nephron number in patients with primary hypertension. N. Engl. J. Med. 348, 101–108 (2003).
Hartman, H. A., Lai, H. L. & Patterson, L. T. Cessation of renal morphogenesis in mice. Dev. Biol. 310, 379–387 (2007).
Fogo, A. & Ichikawa, I. Evidence for a pathogenic linkage between glomerular hypertrophy and sclerosis. Am. J. Kidney Dis. 17, 666–669 (1991).
Bertram, J. F. et al. Why and how we determine nephron number. Pediatr. Nephrol. 29, 575–580 (2014).
Chen, J. S. et al. Mouse model of membranous nephropathy induced by cationic bovine serum albumin: antigen dose-response relations and strain differences. Nephrol. Dial. Transplant. 19, 2721–2728 (2004).
Border, W. A., Ward, H. J., Kamil, E. S. & Cohen, A. H. Induction of membranous nephropathy in rabbits by administration of an exogenous cationic antigen. J. Clin. Investig. 69, 451–461 (1982).
Cloutier, N. et al. Platelets release pathogenic serotonin and return to circulation after immune complex-mediated sequestration. Proc. Natl Acad. Sci. USA 115, E1550–E1559 (2018).
Acknowledgements
We thank Molecular Imaging Core (Rene “Jack” Roy), Advanced Microscopy Core, and ACUC (Sanford Feldman, Gina Wimer, and Jeremy Gatesman). This work was supported by The Hartwell Foundation and NIH/NIDDK: R01DK110622 and R01DK111861. This work used the Bruker ClinScan 7 T MRI in the Molecular Imaging Core, which was purchased with support from NIH grant 1S10RR019911-01 and is supported by the University of Virginia School of Medicine.
Author information
Authors and Affiliations
Contributions
J.R.C. and K.M.B.: designed the study and drafted and revised the paper; J.R.C., E.J.B., K.d., S.C., D.H., S.N., V.P.: carried out experiments; J.R.C., E.J.B., H.C., N.P.C., K.M.B.: analyzed the data; and all authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
J.R.C. and K.M.B. are co-owners of Sindri Technologies, LLC.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Charlton, J.R., Baldelomar, E.J., deRonde, K.A. et al. Nephron loss detected by MRI following neonatal acute kidney injury in rabbits. Pediatr Res 87, 1185–1192 (2020). https://doi.org/10.1038/s41390-019-0684-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-019-0684-1
This article is cited by
-
Windows of susceptibility to neonatal acute kidney injury and nephron loss in a rabbit model
Scientific Reports (2025)
-
Expanded discussion of kidney health monitoring for critically ill term and late preterm infants after acute kidney injury: a report from the Neonatal Kidney Health Consensus Workshop
Pediatric Nephrology (2025)
-
Use of novel structural features to identify urinary biomarkers during acute kidney injury that predict progression to chronic kidney disease
BMC Nephrology (2023)
-
The impact of intrauterine growth restriction and prematurity on nephron endowment
Nature Reviews Nephrology (2023)
-
Acute kidney injury decreases pulmonary vascular growth and alveolarization in neonatal rat pups
Pediatric Research (2023)