Abstract
Background
There is an association between hypocapnia and adverse neurodevelopmental outcome in infants with neonatal encephalopathy (NE). Our aim was to test the safety and feasibility of 5% CO2 and 95% air inhalation to correct hypocapnia in mechanically ventilated infants with NE undergoing therapeutic hypothermia.
Methods
Ten infants were assigned to this open-label, single-center trial. The gas mixture of 5% CO2 and 95% air was administered through patient circuits if the temperature-corrected PCO2 ≤40 mm Hg. The CO2 inhalation was continued for 12 h or was stopped earlier if the base deficit (BD) level decreased <5 mmol/L. Follow-up was performed using Bayley Scales of Infant Development II.
Results
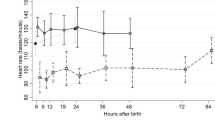
The patients spent a median 95.1% (range 44.6–98.5%) of time in the desired PCO2 range (40–60 mm Hg) during the inhalation. All PCO2 values were >40 mm Hg, the lower value of the target range. Regression modeling revealed that BD and lactate had a tendency to decrease during the intervention (by 0.61 and 0.55 mmol/L/h, respectively), whereas pH remained stable. The rate of moderate disabilities and normal outcome was 50%.
Conclusions
Our results suggest that inhaled 5% CO2 administration is a feasible and safe intervention for correcting hypocapnia.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Lawn, J. E., Kerber, K., Enweronu-Laryea, C. & Cousens, S. 3.6 million neonatal deaths-what is progressing and what is not? Semin. Perinatol. 34, 371–386 (2010).
Shankaran, S. et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N. Engl. J. Med. 366, 2085–2092 (2012).
Edwards, A. D. et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ 340, c363 (2010).
Pappas, A. et al. Hypocarbia and adverse outcome in neonatal hypoxic-ischemic encephalopathy. J. Pediatrics 158, 752–8 e1 (2011).
Lingappan, K., Kaiser, J. R., Srinivasan, C. & Gunn, A. J. Relationship between PCO2 and unfavorable outcome in infants with moderate-to-severe hypoxic ischemic encephalopathy. Pediatr. Res. 80, 204–208 (2016).
Klinger, G., Beyene, J., Shah, P. & Perlman, M. Do hyperoxaemia and hypocapnia add to the risk of brain injury after intrapartum asphyxia? Arch. Dis. Child. Fetal Neonatal Ed. 90, F49–F52 (2005).
Nadeem, M., Murray, D., Boylan, G., Dempsey, E. M. & Ryan, C. A. Blood carbon dioxide levels and adverse outcome in neonatal hypoxic-ischemic encephalopathy. Am. J. Perinatol. 27, 361–365 (2010).
Szakmar, E. et al. Asphyxiated neonates who received active therapeutic hypothermia during transport had higher rates of hypocapnia than controls. Acta Paediatr. 107, 1902–1908 (2018).
Lasso Pirot, A., Fritz, K. I., Ashraf, Q. M., Mishra, O. P. & Delivoria-Papadopoulos, M. Effects of severe hypocapnia on expression of bax and bcl-2 proteins, DNA fragmentation, and membrane peroxidation products in cerebral cortical mitochondria of newborn piglets. Neonatology 91, 20–27 (2007).
Vannucci, R. C., Towfighi, J., Heitjan, D. F. & Brucklacher, R. M. Carbon dioxide protects the perinatal brain from hypoxic-ischemic damage: an experimental study in the immature rat. Pediatrics 95, 868–874 (1995).
Greisen, G., Munck, H. & Lou, H. Severe hypocarbia in preterm infants and neurodevelopmental deficit. Acta Paediatr. Scand. 76, 401–404 (1987).
Calvert, S. A., Hoskins, E. M., Fong, K. W. & Forsyth, S. C. Etiological factors associated with the development of periventricular leukomalacia. Acta Paediatr. Scand. 76, 254–259 (1987).
Vaahersalo, J. et al. Arterial blood gas tensions after resuscitation from out-of-hospital cardiac arrest: associations with long-term neurologic outcome. Crit. Care Med. 42, 1463–1470 (2014).
Schneider, A. G. et al. Arterial carbon dioxide tension and outcome in patients admitted to the intensive care unit after cardiac arrest. Resuscitation 84, 927–934 (2013).
Laffey, J. G. & Kavanagh, B. P. Hypocapnia. N. Engl. J. Med. 347, 43–53 (2002).
Kraut, J. A. & Madias, N. E. Metabolic acidosis: pathophysiology, diagnosis and management. Nat. Rev. Nephrol. 6, 274–285 (2010).
Zanelli, S., Buck, M. & Fairchild, K. Physiologic and pharmacologic considerations for hypothermia therapy in neonates. J. Perinatol. 31, 377–386 (2011).
Al-Saif, S. et al. A randomized controlled trial of theophylline versus CO2 inhalation for treating apnea of prematurity. J. Pediatrics 153, 513–518 (2008).
Alvaro, R. E. et al. A developmental study of the dose-response curve of the respiratory sensory reflex. Am. Rev. Respir. Dis. 148, 1013–1017 (1993).
Alvaro, R. E. et al. CO(2) inhalation as a treatment for apnea of prematurity: a randomized double-blind controlled trial. J. Pediatrics 160, 252–7 e1 (2012).
Forsyth, R., Martland, T., Lai, M., Vadlamani, G. & Hogan, V. 5% Carbon dioxide is safe but of limited efficacy as a treatment for paediatric non-convulsive status epilepticus: an open label observational study. Eur. J. Paediatr. Neurol. 20, 560–565 (2016).
Ohlraun, S. et al. CARbon DIoxide for the treatment of Febrile seizures: rationale, feasibility, and design of the CARDIF-study. J. Transl. Med. 11, 157 (2013).
Rigatto, H., Brady, J. P., de la Torre & Verduzco, R. Chemoreceptor reflexes in preterm infants: II. The effect of gestational and postnatal age on the ventilatory response to inhaled carbon dioxide. Pediatrics 55, 614–620 (1975).
Azzopardi, D. V. et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N. Engl. J. Med. 361, 1349–1358 (2009).
Ashwood, E. R., Kost, G. & Kenny, M. Temperature correction of blood-gas and pH measurements. Clin. Chem. 29, 1877–1885 (1983).
Thompson, C. M. et al. The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatr. 86, 757–761 (1997).
Curley, G., Laffey J. G. & Kavanagh B. P. Bench-to-bedside review: carbon dioxide. Crit Care 14, 220 (2010).
Del Castillo, J. et al. Hyperoxia, hypocapnia and hypercapnia as outcome factors after cardiac arrest in children. Resuscitation 83, 1456–1461 (2012).
Kaiser, J. R., Gauss, C. H., Pont, M. M. & Williams, D. K. Hypercapnia during the first 3 days of life is associated with severe intraventricular hemorrhage in very low birth weight infants. J. Perinatol. 26, 279–285 (2006).
Gupta, S. N., Kechli, A. M. & Kanamalla, U. S. Intracranial hemorrhage in term newborns: management and outcomes. Pediatr. Neurol. 40, 1–12 (2009).
Forman, K. R. et al. Coagulopathy in newborns with hypoxic ischemic encephalopathy (HIE) treated with therapeutic hypothermia: a retrospective case-control study. BMC Pediatr. 14, 277 (2014).
Greisen, G. Autoregulation of cerebral blood flow in newborn babies. Early Hum. Dev. 81, 423–428 (2005).
Pryds, O., Greisen, G., Lou, H. & Friis-Hansen, B. Vasoparalysis associated with brain damage in asphyxiated term infants. J. Pediatrics 117, 119–125 (1990).
Haaland, K., Karlsson, B., Skovlund, E., Lagercrantz, H. & Thoresen, M. Postnatal development of the cerebral blood flow velocity response to changes in CO2 and mean arterial blood pressure in the piglet. Acta Paediatr. 84, 1414–20. (1995).
Noori, S., Anderson, M., Soleymani, S. & Seri, I. Effect of carbon dioxide on cerebral blood flow velocity in preterm infants during postnatal transition. Acta Paediatr. 103, e334–e339 (2014).
Pryds, O., Greisen, G., Lou, H. & Friis-Hansen, B. Heterogeneity of cerebral vasoreactivity in preterm infants supported by mechanical ventilation. J. Pediatrics 115, 638–645 (1989).
Berger, A. J., Mitchell, R. A. & Severinghaus, J. W. Regulation of respiration (first of three parts). N. Engl. J. Med. 297, 92–97 (1977).
Cross, K. W., Hooper, J. M. & Oppe, T. E. The effect of inhalation of carbon dioxide in air on the respiration of the full-term and premature infant. J. Physiol. 122, 264–273 (1953).
Al-Aif, S. et al. Inhalation of low (0.5–1.5%) CO2 as a potential treatment for apnea of prematurity. Semin. Perinatol. 25, 100–106 (2001).
Tingay, D. G., Stewart, M. J. & Morley, C. J. Monitoring of end tidal carbon dioxide and transcutaneous carbon dioxide during neonatal transport. Arch. Dis. Child. Fetal Neonatal Ed. 90, F523–F526 (2005).
Acknowledgements
We acknowledge the important contribution of Professor Dr. Istvan Seri, Professor of Paediatrics, USC Keck School of Medicine, Los Angeles, CA in discussing the study design and reviewing the paper. We thank Laszlo Szakacs, Planimeter Statistics Ltd., Budapest, Hungary for expert help with data management and statistical analysis. We also thank the medical and nursing team at the NICU of the Semmelweis University, 1st Department of Paediatrics for the professional care and study management. A.J. was supported by the Hungarian Academy of Science, Premium Postdoctoral Fellowship (PPD460004). The funding agency had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Author information
Authors and Affiliations
Contributions
E.S. contributed to the study design, recruited participants, collected the data, carried out the initial analyses, interpreted the data, wrote the initial manuscript draft, and reviewed and revised the manuscript. K.K. helped to collect and analyze the data and to write the manuscript draft. U.M. recruited participants, collected the data, analyzed the aEEG traces, and edited the manuscript. G. Bokodi and C.A. recruited patients, made significant contribution to the interpretation of data, and revised the manuscript. A.L. analyzed MRI studies and edited and revised the manuscript. A.J.S. and G. Belteki supervised the interpretation of data and revised the manuscript for important intellectual content. M.S. and A.J. conceptualized and designed the study, supervised all aspects, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Szakmar, E., Kovacs, K., Meder, U. et al. Neonatal encephalopathy therapy optimization for better neuroprotection with inhalation of CO2: the HENRIC feasibility and safety trial. Pediatr Res 87, 1025–1032 (2020). https://doi.org/10.1038/s41390-019-0697-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-019-0697-9
This article is cited by
-
Carbon dioxide and MAPK signalling: towards therapy for inflammation
Cell Communication and Signaling (2023)
-
Carbon dioxide levels in neonates: what are safe parameters?
Pediatric Research (2022)
-
Hypocapnia in early hours of life is associated with brain injury in moderate to severe neonatal encephalopathy
Journal of Perinatology (2022)
-
Carbon dioxide as a drug in neonatology
Pediatric Research (2021)