Abstract
Background
Uncertainty remains about the role of probiotics to prevent necrotising enterocolitis (NEC) some of which arises from the variety of probiotic interventions used in different trials, many with no prior evidence of potential efficacy. Mechanistic studies of intestinal barrier function embedded in a large probiotic trial could provide evidence about which properties of probiotics might be important for NEC prevention thus facilitating identification of strains with therapeutic potential.
Methods
Intestinal permeability, stool microbiota, SCFAs and mucosal inflammation were assessed from the second postnatal week in babies enrolled to a randomised controlled trial of B. breve BBG-001 (the PiPS trial). Results were compared by allocation and by stool colonisation with the probiotic.
Results
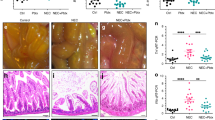
Ninety-four preterm babies were recruited across six nested studies. B. breve BBG-001 content was higher by allocation and colonisation; Enterobacteriaceae and acetic acid levels were higher by colonisation. No measure of intestinal barrier function showed differences. The PiPS trial found no evidence of efficacy to reduce NEC.
Conclusions
That the negative results of the PiPS trial were associated with failure of this probiotic to modify intestinal barrier function supports the possibility that the tests described here have the potential to identify strains to progress to large clinical trials.
Impact
-
Uncertainty about the therapeutic role of probiotics to prevent necrotising enterocolitis is in part due to the wide range of bacterial strains with no previous evidence of efficacy used in clinical trials.
-
We hypothesised that mechanistic studies embedded in a probiotic trial would provide evidence about which properties of probiotics might be important for NEC prevention.
-
The finding that the probiotic strain tested, Bifidobacterium breve BBG-001, showed neither effects on intestinal barrier function nor clinical efficacy supports the possibility that these tests have the potential to identify strains to progress to large clinical trials.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Battersby, C. et al. Incidence and enteral feed antecedents of severe neonatal necrotising enterocolitis across neonatal networks in England, 2012-13: a whole-population surveillance study. Lancet Gastroenterol. Hepatol. 2, 43–51 (2017).
Neu, J. & Walker, W. A. Necrotizing enterocolitis. N. Engl. J. Med. 364, 255–264 (2011).
Alganabi, M., Lee, C., Bindi, E., Li, B. & Pierro, A. Recent advances in understanding necrotizing enterocolitis. F1000Res 8, 107 (2019).
Battersby, C., Santhalingam, T., Costeloe, K. & Modi, N. Incidence of neonatal necrotising enterocolitis in high-income countries: a systematic review. Arch. Dis. Child. Fetal Neonatal Ed. 103, F182–F189 (2018).
Hall, N. J., Eaton, S. & Pierro, A. Royal Australasia of Surgeons Guest Lecture. Necrotizing enterocolitis: prevention, treatment, and outcome. J. Pediatr. Surg. 48, 2359–2367 (2013).
Dani, C., Biadaioli, R., Bertini, G., Martelli, E. & Rubaltelli, F. F. Probiotics feeding in prevention of urinary tract infection, bacterial sepsis and necrotizing enterocolitis in preterm infants. A prospective double-blind study. Biol. Neonate 82, 103–108 (2002).
Lin, H. C. et al. Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics 115, 1–4 (2005).
Bin-Nun, A. et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J. Pediatr. 147, 192–196 (2005).
Lin, H. C. et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial. Pediatrics 122, 693–700 (2008).
Jacobs, S. E. et al. Probiotic effects on late-onset sepsis in very preterm infants: a randomized controlled trial. Pediatrics 132, 1055–1062 (2013).
Sawh, S. C., Deshpande, S., Jansen, S., Reynaert, C. J. & Jones, P. M. Prevention of necrotizing enterocolitis with probiotics: a systematic review and meta-analysis. PeerJ 4, e2429 (2016).
Jarrett, P., Meczner, A., Costeloe, K. & Fleming, P. Historical aspects of probiotic use to prevent necrotising enterocolitis in preterm babies. Early Hum. Dev. 135, 51–57 (2019).
Costeloe, K. et al. Bifidobacterium breve BBG-001 in very preterm infants: a randomised controlled phase 3 trial. Lancet 387, 649–660 (2016).
Piena-Spoel, M., Albers, M. J., ten Kate, J. & Tibboel, D. Intestinal permeability in newborns with necrotizing enterocolitis and controls: does the sugar absorption test provide guidelines for the time to (re-)introduce enteral nutrition?. J. Pediatr. Surg. 36, 587–592 (2001).
Weaver, L. T., Laker, M. F. & Nelson, R. Enhanced intestinal permeability in preterm babies with bloody stools. Arch. Dis. Child. 59, 280–281 (1984).
Shulman, R. J., Buffone, G. & Wise, L. Enteric protein loss in necrotizing enterocolitis as measured by fecal alpha 1-antitrypsin excretion. J. Pediatr. 107, 287–289 (1985).
Sharma, R. et al. Neonatal gut barrier and multiple organ failure: role of endotoxin and proinflammatory cytokines in sepsis and necrotizing enterocolitis. J. Pediatr. Surg. 42, 454–461 (2007).
Beach, R. C., Menzies, I. S., Clayden, G. S. & Scopes, J. W. Gastrointestinal permeability changes in the preterm neonate. Arch. Dis. Child. 57, 141–145 (1982).
Weaver, L. T., Laker, M. F. & Nelson, R. Intestinal permeability in the newborn. Arch. Dis. Child. 59, 236–241 (1984).
Shulman, R. J. et al. Early feeding, antenatal glucocorticoids, and human milk decrease intestinal permeability in preterm infants. Pediatr. Res. 44, 519–523 (1998).
Rouwet, E. V. et al. Intestinal permeability and carrier-mediated monosaccharide absorption in preterm neonates during the early postnatal period. Pediatr. Res. 51, 64–70 (2002).
van Elburg, R. M., Fetter, W. P., Bunkers, C. M. & Heymans, H. S. Intestinal permeability in relation to birth weight and gestational and postnatal age. Arch. Dis. Child. Fetal Neonatal Ed. 88, F52–F55 (2003).
Warner, B. B. et al. Gut bacteria dysbiosis and necrotising enterocolitis in very low birthweight infants: a prospective case-control study. Lancet 387, 1928–1936 (2016).
Wang, C. et al. Effects of oral administration of Bifidobacterium breve on fecal lactic acid and short-chain fatty acids in low birth weight infants. J. Pediatr. Gastroenterol. Nutr. 44, 252–257 (2007).
Thuijls, G. et al. Non-invasive markers for early diagnosis and determination of the severity of necrotizing enterocolitis. Ann. Surg. 251, 1174–1180 (2010).
Stratiki, Z. et al. The effect of a bifidobacter supplemented bovine milk on intestinal permeability of preterm infants. Early Hum. Dev. 83, 575–579 (2007).
Kitajima, H. et al. Early administration of Bifidobacterium breve to preterm infants: randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 76, F101–F107 (1997).
Mohan, R. et al. Effects of Bifidobacterium lactis Bb12 supplementation on body weight, fecal pH, acetate, lactate, calprotectin, and IgA in preterm infants. Pediatr. Res. 64, 418–422 (2008).
Goncalves, F. L. et al. Evaluation of the expression of I-FABP and L-FABP in a necrotizing enterocolitis model after the use of Lactobacillus acidophilus. J. Pediatr. Surg. 50, 543–549 (2015).
Costeloe, K. et al. A randomised controlled trial of the probiotic Bifidobacterium breve BBG-001 in preterm babies to prevent sepsis, necrotising enterocolitis and death: the Probiotics in Preterm infantS (PiPS) trial. Health Technol. Assess. 20, 1–194 (2016).
Jiang, W. et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J. Infect. Dis. 199, 1177–1185 (2009).
Matsuki, T. et al. Development of 16S rRNA-gene-targeted group-specific primers for the detection and identification of predominant bacteria in human feces. Appl. Environ. Microbiol. 68, 5445–5451 (2002).
Matsuki, T., Watanabe, K., Fujimoto, J., Takada, T. & Tanaka, R. Use of 16S rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in human feces. Appl. Environ. Microbiol. 70, 7220–7228 (2004).
Kanazawa, H. et al. Synbiotics reduce postoperative infectious complications: a randomized controlled trial in biliary cancer patients undergoing hepatectomy. Langenbecks Arch. Surg. 390, 104–113 (2005).
Underwood, M. A. et al. A randomized placebo-controlled comparison of 2 prebiotic/probiotic combinations in preterm infants: impact on weight gain, intestinal microbiota, and fecal short-chain fatty acids. J. Pediatr. Gastroenterol. Nutr. 48, 216–225 (2009).
Keller, K. M., Knobel, R. & Ewe, K. Fecal alpha 1-antitrypsin in newborn infants. J. Pediatr. Gastroenterol. Nutr. 24, 271–275 (1997).
Majamaa, H. & Isolauri, E. Probiotics: a novel approach in the management of food allergy. J. Allergy Clin. Immunol. 99, 179–185 (1997).
Vergnano, S. et al. Neonatal infections in England: the NeonIN surveillance network. Arch. Dis. Child. Fetal Neonatal Ed. 96, F9–F14 (2011).
Taylor, S. N., Basile, L. A., Ebeling, M. & Wagner, C. L. Intestinal permeability in preterm infants by feeding type: mother’s milk versus formula. Breastfeed. Med. 4, 11–15 (2009).
Westerbeek, E. A. et al. The effect of enteral supplementation of specific neutral and acidic oligosaccharides on the faecal microbiota and intestinal microenvironment in preterm infants. Eur. J. Clin. Microbiol. Infect. Dis. 32, 269–276 (2013).
Bischoff, S. C. et al. Intestinal permeability-a new target for disease prevention and therapy. BMC Gastroenterol. 14, 189 (2014).
Munford, R. S. Endotoxemia-menace, marker, or mistake?. J. Leukoc. Biol. 100, 687–698 (2016).
Berrington, J. E., Stewart, C. J., Embleton, N. D. & Cummings, S. P. Gut microbiota in preterm infants: assessment and relevance to health and disease. Arch. Dis. Child. Fetal Neonatal Ed. 98, F286–F290 (2013).
Drell, T. et al. The development of gut microbiota in critically ill extremely low birth weight infants assessed with 16S rRNA gene based sequencing. Gut Microbes 5, 304–312 (2014).
Sorbara, M. T. et al. Inhibiting antibiotic-resistant Enterobacteriaceae by microbiota-mediated intracellular acidification. J. Exp. Med. 216, 84–98 (2019).
Millar, M. et al. The microbiome of infants recruited to a randomised placebo-controlled probiotic trial (PiPS Trial). EBioMedicine 20, 255–262 (2017).
van den Akker, C. H. P. et al. Probiotics for preterm infants: a strain-specific systematic review and network meta-analysis. J. Pediatr. Gastroenterol. Nutr. 67, 103–122 (2018).
van den Akker, C. H. P. et al. Probiotics and preterm infants: A Position Paper by the European Society for Paediatric Gastroenterology Hepatology and Nutrition Committee on Nutrition and the European Society for Paediatric Gastroenterology Hepatology and Nutrition Working Group for Probiotics and Prebiotics. J. Pediatr. Gastroenterol. Nutr. 70, 664–680 (2020).
Acknowledgements
Our thanks are extended to Yakult Honsha European Research Centre for Microbiology ESV (YHER), Belgium for analysing faecal samples for both microbiota and short-chain fatty acids. YHER had no role in the data analyses and interpretation of the results presented in this manuscript. The interventions for the PiPS trial were provided without charge by Yakult Honsha Co. Ltd. (Tokyo, Japan) until the point of entry to the UK. The PiPS trial was funded by the UK National Institute for Health Research Health Technology Assessment Programme, project number 05/501/04. Simon Eaton acknowledges support from the NIHR Great Ormond Street Biomedical Research Centre. Special thanks are extended to Mr. Nik Hannay for creating the figure presented in this manuscript. This study was supported by a strategic Research Grant from Barts Charity (Ref: 719/1102).
Author information
Authors and Affiliations
Contributions
P.F. designed the study, performed laboratory analyses, analysed data and wrote the manuscript; M.W. designed the study, provided laboratory support and reviewed the data and manuscript; S.E., N.P. and R.H. performed laboratory analyses and reviewed the manuscript; A.A. assisted with clinical data collection and reviewed the manuscript; P.H. provided statistical advice and reviewed the manuscript; M.R.M. and K.C. designed the study and co-wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics and consent
Ethics approval was granted by the South London REC 2 Committee (Ref 10/H0802/40). Participants were recruited following informed written parental consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Fleming, P., Wilks, M., Eaton, S. et al. Bifidobacterium breve BBG-001 and intestinal barrier function in preterm babies: Exploratory Studies from the PiPS Trial. Pediatr Res 89, 1818–1824 (2021). https://doi.org/10.1038/s41390-020-01135-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-01135-5
This article is cited by
-
Natural and Biological Products Therapeutic Potential in Restoring Gut Microbiome: Mechanistic Insights and Clinical Implications
Probiotics and Antimicrobial Proteins (2025)
-
The microbiome’s hidden influence: preclinical insights into inflammatory responses in necrotizing enterocolitis
Seminars in Immunopathology (2025)
-
Necrotizing enterocolitis: current understanding of the prevention and management
Pediatric Surgery International (2024)
-
Premature neonatal gut microbial community patterns supporting an epithelial TLR-mediated pathway for necrotizing enterocolitis
BMC Microbiology (2021)