Abstract
Background
The prevalence of autism spectrum disorders (ASD) is 5-fold higher in preterm (PT) infants born ≤28 weeks gestational age (GA) as compared to the general population. The relationship between placental pathologic lesions and ASD in PT infants has not been studied.
Objectives
The objective of this study was to determine the association of placental pathology with the occurrence of ASD in PT infants born ≤28 weeks GA.
Study design
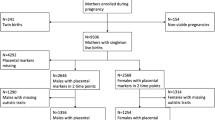
A matched case–control study to identify confirmed ASD cases (n = 16) and matched controls (n = 48) born at Parkland Hospital between January 2012 and December 2015. Patients were matched using known variables associated with increased risk of ASD in PT infants. Placental histology from all births was reviewed.
Results
Children with ASD had 2-fold greater incidence of multiple placental pathologic lesions vs. matched controls [11/16 (69%) vs.16/48 (33%), respectively; P = 0.01]. In contrast, single placental pathologic lesions were not associated with ASD [5/16 (31%) vs. 21/48 (43%), respectively; P = 0.1].
Conclusions
In this study, we have demonstrated an association between the increasing complexity of histologic placental lesions and the later risk for ASD in infants born ≤28 weeks GA. Thus, placental pathology findings may be valuable in further understanding the prenatal pathologic processes underlying ASD in PT infants.
Impact
-
PT infants with ASD have a 2-fold greater incidence of multiple placental pathologies.
-
This is the first study to report an association between the complexity of histologic placental lesions and later risk of ASD in infant born extremely PT (i.e., ≤28 weeks GA).
-
This study reiterates the importance of examining placental pathologic lesions, since placental evidence of antenatal insults correlates with postnatal morbidities and mortality in PT infants.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Levy, S. E., Mandell, D. S. & Schultz, R. T. Autism. Lancet 374, 1627–1638 (2009).
Hack, M. et al. Behavioral outcomes of extremely low birth weight children at age 8 years. J. Dev. Behav. Pediatr. 30, 122–130 (2009).
Johnson, S. et al. Autism spectrum disorders in extremely preterm children. J. Pediatr. 156, 525–31. e2 (2010).
Pinto-Martin, J. A. et al. Prevalence of autism spectrum disorder in adolescents born weighing <2000 grams. Pediatrics 128, 883–891 (2011).
Treyvaud, K. et al. Psychiatric outcomes at age seven for very preterm children: rates and predictors. J. Child Psychol. Psychiatry 54, 772–779 (2013).
Joseph, R. M. et al. Prevalence and associated features of autism spectrum disorder in extremely low gestational age newborns at age 10 years. Autism Res. 10, 224–232 (2017).
Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators; Centers for Disease Control and Prevention (CDC). Prevalence of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill. Summ. 63, 1–21 (2014).
Kuzniewicz, M. W. et al. Prevalence and neonatal factors associated with autism spectrum disorders in preterm infants. J. Pediatr. 164, 20–25 (2014).
Dammann, O. & Leviton, A. Brain damage in preterm newborns: might enhancement of developmentally regulated endogenous protection open a door for prevention? Pediatrics 104, 541–550 (1999).
Leviton, A. et al. The wealth of information conveyed by gestational age. J. Pediatr. 146, 123–127 (2005).
Sanders, E. J. & Harvey, S. Peptide hormones as developmental growth and differentiation factors. Dev. Dyn. 237, 1537–1552 (2008).
Bastek, J. A. et al. Biomarkers of inflammation and placental dysfunction are associated with subsequent preterm birth. J. Matern. Fetal Neonatal Med. 24, 600–605 (2011).
Leviton, A. et al. Relationships among the concentrations of 25 inflammation-associated proteins during the first postnatal weeks in the blood of infants born before the 28th week of gestation. Cytokine 57, 182–90 (2012).
Wei, S. Q., Fraser, W. & Luo, Z. C. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: a systematic review. Obstet. Gynecol. 116, 393–401 (2010).
Lee, B. K. et al. Maternal hospitalization with infection during pregnancy and risk of autism spectrum disorders. Brain Behav. Immun. 44, 100–105 (2015).
Onore, C., Careaga, M. & Ashwood, P. The role of immune dysfunction in the pathophysiology of autism. Brain Behav. Immun. 26, 383–392 (2012).
Rusterholz, C., Hahn, S. & Holzgreve, W. Role of placentally produced inflammatory and regulatory cytokines in pregnancy and the etiology of preeclampsia. Semin. Immunopathol. 29, 151–162 (2007).
Roescher, A. M. et al. Placental pathology and neurological morbidity in preterm infants during the first two weeks after birth. Early Hum. Dev. 90, 21–25 (2014).
Kramer, B. W. et al. Decreased expression of angiogenic factors in placentas with chorioamnionitis after preterm birth. Pediatr. Res. 58, 607–612 (2005).
Roescher, A. M. et al. Placental pathology, perinatal death, neonatal outcome, and neurological development: a systematic review. PLoS ONE 9, e89419 (2014).
Atladottir, H. O. et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J. Autism Dev. Disord. 40, 1423–1430 (2010).
Chess, S. Autism in children with congenital rubella. J. Autism Child Schizophr. 1, 33–47 (1971).
Guinchat, V. et al. Pre-, peri- and neonatal risk factors for autism. Acta Obstet. Gynecol. Scand. 91, 287–300 (2012).
Challier, J. C. et al. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta 29, 274–281 (2008).
Roberts, K. A. et al. Placental structure and inflammation in pregnancies associated with obesity. Placenta 32, 247–254 (2011).
Ghidini, A. & Salafia, C. M. Sex differences of placental dysfunction in severe prematurity. BJOG 112, 140–144 (2005).
Limperopoulos, C. et al. Positive screening for autism in ex-preterm infants: prevalence and risk factors. Pediatrics 121, 758–765 (2008).
Straughen, J. K. et al. The association between placental histopathology and autism spectrum disorder. Placenta 57, 183–188 (2017).
Raghavan, R. et al. Preterm birth subtypes, placental pathology findings, and risk of neurodevelopmental disabilities during childhood. Placenta 83, 17–25 (2019).
Albers, C. A. & Grieve, A. J. Test review: Bayley, N. (2006). Bayley Scales of Infant and Toddler Development—Third Edition. San Antonio, TX: Harcourt Assessment. J. Psychoeduc. Assess. 25, 180–190 (2007).
Vohr, B. R. et al. Are outcomes of extremely preterm infants improving? Impact of Bayley assessment on outcomes. J. Pediatr. 161, 222–8.e3 (2012).
Robins, D. L. et al. The Modified Checklist for Autism in Toddlers: an initial study investigating the early detection of autism and pervasive developmental disorders. J. Autism Dev. Disord. 31, 131–144 (2001).
Lord, C. et al. Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) [Manual: Modules 1-4] (Western Psychological Services, Torrance, 2012).
Chlebowski, C. et al. Using the childhood autism rating scale to diagnose autism spectrum disorders. J. Autism Dev. Disord. 40, 787–799 (2010).
Gardener, H., Spiegelman, D. & Buka, S. L. Prenatal risk factors for autism: comprehensive meta-analysis. Br. J. Psychiatry 195, 7–14 (2009).
Greer, L. G. et al. An immunologic basis for placental insufficiency in fetal growth restriction. Am. J. Perinatol. 29, 533–538 (2012).
Wintermark, P. et al. Placental pathology in asphyxiated newborns meeting the criteria for therapeutic hypothermia. Am. J. Obstet. Gynecol. 203, 579 e1–579 e9 (2010).
Redline, R. W. et al. Placental diagnostic criteria and clinical correlation—a workshop report. Placenta 26, S114–S117 (2005).
Mir, I. N. et al. Placental pathology is associated with severity of neonatal encephalopathy and adverse developmental outcomes following hypothermia. Am. J. Obstet. Gynecol. 213, 849 e1–7 (2015).
Redline, R. W. et al. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr. Dev. Pathol. 6, 435–448 (2003).
Redline, R. W. & Patterson, P. Villitis of unknown etiology is associated with major infiltration of fetal tissue by maternal inflammatory cells. Am. J. Pathol. 143, 473–479 (1993).
Redline, R. W. Villitis of unknown etiology: noninfectious chronic villitis in the placenta. Hum. Pathol. 38, 1439–1446 (2007).
Redline, R. W. et al. Maternal vascular underperfusion: nosology and reproducibility of placental reaction patterns. Pediatr. Dev. Pathol. 7, 237–249 (2004).
Redline, R. W. et al. Fetal vascular obstructive lesions: nosology and reproducibility of placental reaction patterns. Pediatr. Dev. Pathol. 7, 443–452 (2004).
Renaer, M., Van de Putte, I. & Vermylen, C. Massive feto-maternal hemorrhage as a cause of perinatal mortality and morbidity. Eur. J. Obstet. Gynecol. Reprod. Biol. 6, 125–140 (1976).
Lewis, N. E., Marszalek, L. & Ernst, L. M. Placental pathologic features in fetomaternal hemorrhage detected by flow cytometry. Pediatr. Dev. Pathol. 20, 142–151 (2017).
Pinar, H. et al. Reference values for singleton and twin placental weights. Pediatr. Pathol. Lab. Med. 16, 901–907 (1996).
Salafia, C. et al. Characterization of placental growth as a biomarker of Autism/Asd risk. Placenta 33, A16–A16 (2012).
Wu, Y. W. & Colford, J. M. Jr. Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. JAMA 284, 1417–1424 (2000).
Aoki, Y. et al. Association of white matter structure with autism spectrum disorder and attention-deficit/hyperactivity disorder. JAMA Psychiatry 74, 1120–1128 (2017).
Naldini, A. & Carraro, F. Role of inflammatory mediators in angiogenesis. Curr. Drug Targets Inflamm. Allergy 4, 3–8 (2005).
Favrais, G. et al. Systemic inflammation disrupts the developmental program of white matter. Ann. Neurol. 70, 550–565 (2011).
Felderhoff-Mueser, U. et al. Oxygen causes cell death in the developing brain. Neurobiol. Dis. 17, 273–282 (2004).
Ment, L. R. et al. Association of chronic sublethal hypoxia with ventriculomegaly in the developing rat brain. Brain Res. Dev. Brain Res. 111, 197–203 (1998).
Fleiss, B. et al. Inflammation-induced sensitization of the brain in term infants. Dev. Med. Child Neurol. 57, 17–28 (2015).
van Tilborg, E. et al. Combined fetal inflammation and postnatal hypoxia causes myelin deficits and autism-like behavior in a rat model of diffuse white matter injury. Glia 66, 78–93 (2018).
Stein, Z. & Susser, M. The Dutch Famine, 1944–1945, and the reproductive process. II. Interrelations of caloric rations and six indices at birth. Pediatr. Res. 9, 76–83 (1975).
Lumey, L. H. Compensatory placental growth after restricted maternal nutrition in early pregnancy. Placenta 19, 105–111 (1998).
Beischer, N. A., Holsman, M. & Kitchen, W. H. Relation of various forms of anemia to placental weight. Am. J. Obstet. Gynecol. 101, 801–809 (1968).
Thompson, L. P. et al. Placental hypoxia during early pregnancy causes maternal hypertension and placental insufficiency in the hypoxic guinea pig model. Biol. Reprod. 95, 128 (2016).
Kim, S. H. et al. Predictive validity of the Modified Checklist for Autism in Toddlers (M-CHAT) born very preterm. J. Pediatr. 178, 101–107. e2 (2016).
Luyster, R. J. et al. The Modified Checklist for Autism in Toddlers in extremely low gestational age newborns: individual items associated with motor, cognitive, vision and hearing limitations. Paediatr. Perinat. Epidemiol. 25, 366–376 (2011).
Acknowledgements
This work was supported by Departmental Funding from UT Southwestern Medical Center.
Author information
Authors and Affiliations
Contributions
All authors contributed to drafting the article or revising it critically for important intellectual content and final approval of the submitted version. Specifically, I.N.M. participated in concept, study design, sample and data acquisition and interpretation, statistical analysis and drafted the first version of the manuscript, and finalized the manuscript for submission after comments from the other authors. L.S.B. performed the statistical analysis, participated in data interpretation and review, revision of the manuscript, and reviewed the final version. S.P.W., R.H., C.R.R., and L.F.C. participated in concept, study design, data interpretation and review, revision of the manuscript, and participated in finalizing the manuscript after comments from the other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Statement of consent
We had waiver of consent for this retrospective study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mir, I.N., White, S.P., Steven Brown, L. et al. Autism spectrum disorders in extremely preterm infants and placental pathology findings: a matched case–control study. Pediatr Res 89, 1825–1831 (2021). https://doi.org/10.1038/s41390-020-01160-4
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-01160-4
This article is cited by
-
Identifying the risk factors of autism spectrum disorders in infants born preterm: a systematic review and meta-analysis
European Child & Adolescent Psychiatry (2026)
-
A validated NICU database: recounting 50 years of clinical growth, quality improvement and research
Pediatric Research (2025)
-
Impact of maternal infection on outcomes in extremely preterm infants
Pediatric Research (2024)
-
The prevalence and profile of autism in individuals born preterm: a systematic review and meta-analysis
Journal of Neurodevelopmental Disorders (2021)
-
Placental origins of neonatal diseases: toward a precision medicine approach
Pediatric Research (2021)