Abstract
Background
Prone sleeping is used in preterm infants undergoing intensive care to improve respiratory function, but evidence suggests that this position may compromise autonomic cardiovascular control. To test this hypothesis, this study assessed the effects of the prone sleeping position on cardiovascular control in preterm infants undergoing intensive care treatment during early postnatal life.
Methods
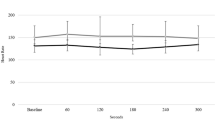
Fifty-six preterm infants, divided into extremely preterm (gestational age (GA) 24–28 weeks, n = 23) and very preterm (GA 29–34 weeks, n = 33) groups, were studied weekly for 3 weeks in prone and supine positions, during quiet and active sleep. Heart rate (HR) and non-invasive blood pressure (BP) were recorded and autonomic measures of HR variability (HRV), BP variability (BPV), and baroreflex sensitivity (BRS) using frequency analysis in low (LF) and high (HF) bands were assessed.
Results
During the first 3 weeks, prone sleeping increased HR, reduced BRS, and increased HF BPV compared to supine. LF and HF HRV were also lower prone compared to supine in very preterm infants. Extremely preterm infants had the lowest HRV and BRS measures, and the highest HF BPV.
Conclusions
Prone sleeping dampens cardiovascular control in early postnatal life in preterm infants, having potential implications for BP regulation in infants undergoing intensive care.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Andriessen, P. et al. Noninvasive assessment of blood pressure variability in preterm infants. Pediatr. Res. 55, 220–223 (2004).
Hsu, K. H. et al. Hemodynamic reference for neonates of different age and weight: a pilot study with electrical cardiometry. J. Perinatol. 36, 481–485 (2016).
Yiallourou, S. R., Witcombe, N. B., Sands, S. A., Walker, A. M. & Horne, R. S. The development of autonomic cardiovascular control is altered by preterm birth. Early Hum. Dev. 89, 145–152 (2013).
Seri, I. Management of hypotension and low systemic blood flow in the very low birth weight neonate during the first postnatal week. J. Perinatol 26(Suppl 1), S8–S13 (2006).
Wong, F. Y. et al. Impaired autoregulation in preterm infants identified by using spatially resolved spectroscopy. Pediatrics 121, e604–e611 (2008).
Wong, F. Y., Silas, R., Hew, S., Samarasinghe, T. & Walker, A. M. Cerebral oxygenation is highly sensitive to blood pressure variability in sick preterm infants. PLoS ONE 7, e43165 (2012).
Volpe, J. J. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 8, 110–124 (2009).
Dionne, J. M. & Flynn, J. T. Management of severe hypertension in the newborn. Arch. Dis. Child. 102, 1176–1179 (2017).
Rivas-Fernandez, M., Roque, I. F. M., Diez-Izquierdo, A., Escribano, J. & Balaguer, A. Infant position in neonates receiving mechanical ventilation. Cochrane Database Syst. Rev. 11, CD003668 (2016).
Yiallourou, S. R., Walker, A. M. & Horne, R. S. C. Effects of sleeping position on development of infant cardiovascular control. Arch. Dis. Child. 93, 868–872 (2008).
Wong, F. Y. et al. Cerebral oxygenation is depressed during sleep in healthy term infants when they sleep prone. Pediatrics 127, E558–E565 (2011).
Yiallourou, S. R., Walker, A. M. & Horne, R. S. Prone sleeping impairs circulatory control during sleep in healthy term infants: implications for SIDS. Sleep 31, 1139–1146 (2008).
Wong, F. et al. Cerebrovascular control is altered in healthy term infants when they sleep prone. Sleep 36, 1911–1918 (2013).
Fyfe, K. L. et al. Cerebral oxygenation in preterm infants. Pediatrics 134, 435–445 (2014).
Fyfe, K. L. et al. Gestational age at birth affects maturation of baroreflex control. J. Pediatr. 166, 559–565 (2015).
Shepherd, K. L. et al. When does prone sleeping improve cardiorespiratory status in preterm infants in the NICU? Sleep 43, zsz256 (2019).
Shepherd, K. L. et al. Effects of prone sleeping on cerebral oxygenation in preterm infants. J. Pediatr. 204, 103–110 (2019).
Sahni, R. et al. Body position, sleep states, and cardiorespiratory activity in developing low birth weight infants. Early Hum. Dev. 54, 197–206 (1999).
Ammari, A. et al. Effects of body position on thermal, cardiorespiratory and metabolic activity in low birth weight infants. Early Hum. Dev. 85, 497–501 (2009).
Fifer, W. P. et al. Interactions between sleeping position and feeding on cardiorespiratory activity in preterm infants. Dev. Psychobiol. 47, 288–296 (2005).
Jean-Louis, M. et al. Power spectral analysis of heart rate in relation to sleep position. Biol. Neonate 86, 81–84 (2004).
Yiallourou, S. R., Walker, A. M. & Horne, R. S. Validation of a new noninvasive method to measure blood pressure and assess baroreflex sensitivity in preterm infants during sleep. Sleep 29, 1083–1088 (2006).
Curzi-Dascalova, L. & Mirmiran, M. Manual of Methods of Recording and Analyzing Sleep-Wakefulness States in Preterm and Full-term Infants (INSERM, Paris, 1996).
Scher, M. S. et al. Computer classification of sleep in preterm and full-term neonates at similar postconceptional term ages. Sleep 19, 18–25 (1996).
Fyfe, K. L. et al. The effect of gestational age at birth on post-term maturation of heart rate variability. Sleep 38, 1635–1644 (2015).
Yiallourou, S. R., Sands, S. A., Walker, A. M. & Horne, R. S. C. Postnatal development of baroreflex sensitivity in infancy. J. Physiol. 588, 2193–2203 (2010).
Yiallourou, S. R., Sands, S. A., Walker, A. M. & Horne, R. S. C. Baroreflex sensitivity during sleep in infants: impact of sleeping position and sleep state. Sleep 34, 725–732 (2011).
Witcombe, N. B., Yiallourou, S. R., Sands, S. A., Walker, A. M. & Horne, R. S. Preterm birth alters the maturation of baroreflex sensitivity in sleeping infants. Pediatrics 129, e89–e96 (2012).
Yiallourou, S. R. et al. Sleep: a window into autonomic control in children born preterm and growth restricted. Sleep 40, zsx048 (2017).
Pagani, M. et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circul. Res. 59, 178–193 (1986).
Malliani, A., Lombardi, F. & Pagani, M. Power spectrum analysis of heart rate variability: a tool to explore neural regulatory mechanisms. Br. Heart J. 71, 1–2 (1994).
Cohen, E. et al. Fetal-growth-restricted preterm infants display compromised autonomic cardiovascular control on the first postnatal day but not during infancy. Pediatr. Res. 82, 474–482 (2017).
Reulecke, S., Schulz, S. & Voss, A. Autonomic regulation during quiet and active sleep states in very preterm neonates. Front. Physiol. 3, 61 (2012).
Goto, K. et al. More awakenings and heart rate variability during supine sleep in preterm infants. Pediatrics 103, 603–609 (1999).
Metsala, T., Siimes, A., Antila, K. & Valimaki, I. Association of breathing movements to the variability of heart rate and blood pressure in foetal lambs. Acta Physiol. Scand. 147, 213–219 (1993).
Sahni, R. et al. Postural differences in cardiac dynamics during quiet and active sleep in low birthweight infants. Acta Paediatr. 88, 1396–1401 (1999).
Skadberg, B. T. & Markestad, T. Behaviour and physiological responses during prone and supine sleep in early infancy. Arch. Dis. Child. 76, 320–324 (1997).
Chong, A., Murphy, N. & Matthews, T. Effect of prone sleeping on circulatory control in infants. Arch. Dis. Child. 82, 253–256 (2000).
Ma, M. et al. Prone positioning decreases cardiac output and increases systemic vascular resistance in neonates. J. Perinatol. 35, 424–427 (2015).
Wu, T. W., Lien, R. I., Seri, I. & Noori, S. Changes in cardiac output and cerebral oxygenation during prone and supine sleep positioning in healthy term infants. Arch. Dis. Child Fetal Neonatal Ed. 102, F483–F489 (2017).
Golder, V., Hepponstall, M., Yiallourou, S. R., Odoi, A. & Horne, R. S. Autonomic cardiovascular control in hypotensive critically ill preterm infants is impaired during the first days of life. Early Hum. Dev. 89, 419–423 (2013).
Van Ravenswaaij-Arts, C., Hopman, J., Kollee, L., Stoelinga, G., Van & Geijn, H. Spectral analysis of heart rate variability in spontaneously breathing very preterm infants. Acta Paediatr. 83, 473–480 (1994).
Gournay, V., Drouin, E. & Roze, J. Development of baroreflex control of heart rate in preterm and full term infants. Arch. Dis. Child. Fetal Neonatal Ed. 86, F151–F154 (2002).
Mazursky, J. E., Birkett, C. L., Bedell, K. A., Ben-Haim, S. A. & Segar, J. L. Development of baroreflex influences on heart rate variability in preterm infants. Early Hum. Dev. 53, 37–52 (1998).
Andriessen, P. et al. Baroreceptor reflex sensitivity in human neonates: the effect of postmenstrual age. J. Physiol. 568(Pt 1), 333–341 (2005).
Blanco, C. E., Dawes, G. S., Hanson, M. A. & McCooke, H. B. Carotid baroreceptors in fetal and newborn sheep. Pediatr. Res. 24, 342–346 (1988).
Segar, J. L. Ontogeny of the arterial and cardiopulmonary baroreflex during fetal and postnatal life. Am. J. Physiol. 273(2 Pt 2), R457–R471 (1997).
Acknowledgements
We would like to thank all the parents and their infants who participated in the study, and the nursing staff of Monash Newborn where the studies were carried out. We especially would like to acknowledge Dr. Nadine Brew, Ph.D., Ms. Stacey Willis, Ms. Kristy Elsayed, Dr. Hannah Cooney, Dr. Brenda Thonissen, and Dr. Margy Zuluaga Fernandez for their help with data acquisition and preliminary analysis. This work was supported by the project grant funding from the National Health and Medical Research Council (NHMRC) of Australia (Project No. 1083026), The Scottish Cot Death Trust, Clive and Vera Ramaciotti Foundation, and the Victorian Government’s Operational Infrastructure Support Program; Dr. Kelsee Shepherd is supported by an Australian Government Research Training Program Scholarship; A/Prof Flora Wong is supported by NHMRC Career Development Fellowships 1084254 and 1159120. Dr. Stephanie Yiallourou is supported by the Alice Baker and Eleanor Shaw Gender Equity Fellowship.
Author information
Authors and Affiliations
Contributions
K.S. contributed to acquisition of data, data analysis, interpretation of data, and drafting of the manuscript; S.Y., F.W., and R.S.C.H. made substantial contributions to conception and design, acquisition of data and interpretation of data, and drafting of the manuscript; E.Y. made contributed to acquisition of data and A.O. contributed to the acquisition of data and analysis. All authors contributed to revising the manuscripts critically for important intellectual content, and final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
Written informed parental consent was obtained for all infants studied and approved by the Monash Health and Monash University human research ethics committees.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shepherd, K.L., Wong, F.Y., Odoi, A. et al. Prone sleeping affects cardiovascular control in preterm infants in NICU. Pediatr Res 90, 197–204 (2021). https://doi.org/10.1038/s41390-020-01254-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-01254-z