Abstract
Background
Noonan Syndrome with Multiple Lentigines (NSML) and Noonan Syndrome (NS) can be difficult to differentiate clinically in early childhood. This study aims to describe characteristics of the ventricular septum that may differentiate NSML from NS. We hypothesize that the shape of the ventricular septum determined by echocardiography correlates with genotype and may distinguish patients with NSML from those with NS.
Methods
We analyzed data from 17 NSML and 67 NS patients. Forty normal and 30 sarcomeric hypertrophic cardiomyopathy (HCM) patients were included as controls. Septal morphology was qualitatively evaluated, and septal angle was measured quantitatively at end diastole. We recorded the presence of a ventricular septal bulge (VSB) and reviewed genetic testing results for each patient.
Results
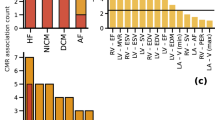
The most important findings were a sigmoid septum (71%) and VSB (71%) in NSML. NSML septal angle was decreased compared to the normal and sarcomeric HCM control groups, respectively (149 ± 13 vs. 177 ± 3, p < 0.001; 149 ± 13 vs. 172 ± 7, p < 0.001). NS septal angle was similar to the controls (176 ± 6 vs. 177 ± 3, p > 0.5; 176 ± 6 vs. 172 ± 7, p > 0.5). NSML-linked pathogenic variants were associated with sigmoid septum and VSB.
Conclusions
These findings provide novel phenotypic evidence to clinicians that may offer incremental diagnostic value in counseling families in ambiguous NSML/NS cases.
Impact
-
Characteristics of the ventricular septum are linked to specific gene variants that cause NSML and NS.
-
Sigmoid septum and VSB are associated with NSML.
-
This novel echocardiographic association may help clinicians distinguish NSML from NS in ambiguous cases.
-
Early distinction between the two may be important, as syndrome-specific therapies may become available in the near future.
-
This study may encourage further research into genotype–phenotype associations in other forms of HCM.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Gorlin, R. J., Anderson, R. C. & Moller, J. H. The LEOPARD (multiple lentigines) syndrome revisited. Birth Defects Orig. Artic. Ser. 7, 110–115 (1971).
Grant, A. R. et al. Assessing the gene‐disease association of 19 genes with the RASopathies using the ClinGen gene curation framework. Hum. Mutat. 39, 1485–1493 (2018).
Gelb, B. D. & Tartaglia, M. Noonan Syndrome with Multiple Lentigines. GeneReviews® [Internet] (eds Adam, M. P. et al.) (University of Washington, Seattle, 2007).
Sarkozy, A., Digilio, M. & Dallapiccola, B. LEOPARD syndrome. Orphanet J. Rare Dis. 3, 13 (2008).
Feng, G. S. & Pawson, T. Phosphotyrosine phosphatases with SH2 domains: regulators of signal transduction. Trends Genet. 10, 54–58 (1994).
Nakamura, T., Gulick, J., Pratt, R. & Robbins, J. Noonan Syndrome is associated with enhanced pERK activity, the repression of which can prevent craniofacial malformations. Proc. Natl Acad. Sci. USA 106, 15436–15441 (2009).
Calcagni, G., Digilio, M. C., Marino, B. & Tartaglia, M. Pediatric patients with RASopathy-associated hypertrophic cardiomyopathy: the multifaceted consequences of PTPN11 mutations. Orphanet J. Rare Dis. 14, 163 (2019).
Hanna, N. et al. Reduced phosphatase activity of SHP-2 in LEOPARD Syndrome: Consequences for PI3K binding on Gab1. FEBS Lett. 580, 2477–2482 (2006).
Hahn, A. et al. Rapidly progressive hypertrophic cardiomyopathy in an infant with Noonan syndrome with multiple lentigines: palliative treatment with a rapamycin analog. Am. J. Med. Genet. A. 167a, 744–751 (2015).
Wang, J. et al. In vivo efficacy of the AKT inhibitor ARQ 092 in Noonan syndrome with multiple lentigines-associated hypertrophic cardiomyopathy. PLoS ONE 12, e0178905 (2017).
Gross A. et al. Advancing RAS/RASopathy therapies: an NCI-sponsored intramural and extramural collaboration for the study of RASopathies. Am. J. Med. Genet. A 182, 866–876 (2020).
Tartaglia, M., Gelb, B. D. & Zenker, M. Noonan syndrome and clinically related disorders. Best Pract. Res. Clin. Endocrinol. Metab. 25, 161–179 (2011).
Tartaglia, M. et al. PTPN11 mutations in Noonan Syndrome: molecular spectrum, genotype-phenotype correlation, and phenotypic heterogeneity. Am. J. Hum. Genet. 70, 1555–1563 (2002).
Binder, J. et al. Echocardiography-guided genetic testing in hypertrophic cardiomyopathy: septal morphological features predict the presence of myofilament mutations. Mayo Clin. Proc. 81, 459–467 (2006).
Neubaur, S. et al. Distinct subgroups in hypertrophic cardiomyopathy in the NHLBI HCM Registry. J. Am. Coll. Cardiol. 74, 2333–2345 (2019).
Tartaglia, M. et al. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat. Genet. 29, 465–468 (2001).
Sarkozy, A. et al. Correlation between PTPN11 gene mutations and congenital heart defects in Noonan and LEOPARD syndromes. J. Med. Genet. 40, 704–708 (2003).
Lee, C. L. et al. Cardiac manifestations and gene mutations of patients with RASopathies in Taiwan. Am. J. Med. Genet. 182, 357–364 (2020).
Krenz, M., Yutzey, K. E. & Robbins, J. Noonan syndrome mutation Q79R in Shp2 increases proliferation of valve primordia mesenchymal cells via extracellular signal-regulated kinase 1/2 signaling. Circ. Res. 97, 813–820 (2005).
Schramm, C., Fine, D. M., Edwards, M. A., Reeb, A. N. & Krenz, M. The PTPN11 loss-of-function mutation Q510E-Shp2 causes hypertrophic cardiomyopathy by dysregulating mTOR signaling. Am. J. Physiol. Heart Circ. Physiol. 302, H231–H243 (2012).
Gelb, B. D., Roberts, A. E. & Tartaglia, M. Cardiomyopathies in Noonan syndrome and the other RASopathies. Prog. Pediatr. Cardiol. 39, 13–19 (2015).
Marin, T. M. et al. Rapamycin reverses hypertrophic cardiomyopathy in a mouse model of LEOPARD syndrome-associated PTPN11 mutation. J. Clin. Investig. 121, 1026–1043 (2011).
WU, X. et al. MEK-ERK pathway modulation ameliorates disease phenotypes in a mouse model of Noonan syndrome associated with the Raf1(L613V) mutation. J. Clin. Investig. 121, 1009–1025 (2011).
Andelfinger, G. et al. Hypertrophic cardiomyopathy in noonan syndrome treated by MEK-inhibition. J. Am. Coll. Cardiol. 73, 2237 (2019).
Calcagni, G., Digilio, M. C., Marino, B. & Tartaglia, M. Pediatric patients with RASopathy-associated hypertrophic cardiomyopathy: the multifaceted consequences of PTPN11 mutations. Orphanet J. Rare Dis. 14, 163 (2019).
Calcagni, G. et al. Clinical presentation and natural history of hypertrophic cardiomyopathy in RASopathies. Heart Fail. Clin. 14, 225–235 (2018).
Theis, J. L. et al. Echocardiographic-determined septal morphology in Z-disc hypertrophic cardiomyopathy. Biochem. Biophys. Res. Commun. 351, 896–902 (2006).
Bos, J. M., Towbin, J. A. & Ackerman, M. J. Diagnostic, prognostic, and therapeutic implications of genetic testing for hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 54, 3 (2009).
Author information
Authors and Affiliations
Contributions
Each author has met the Pediatric Research authorship requirements.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent
This is a retrospective study for which consent is not required by the institutional review board of the Children’s Hospital of Philadelphia.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kauffman, H., Ahrens-Nicklas, R.C., Calderon-Anyosa, R.J.C. et al. Genotype–phenotype association by echocardiography offers incremental value in patients with Noonan Syndrome with Multiple Lentigines. Pediatr Res 90, 444–451 (2021). https://doi.org/10.1038/s41390-020-01292-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-01292-7