Abstract
Background
Volumes of cerebellar posterior lobes have been associated with cognitive skills, such as language functioning. Children born very preterm (VPT) often have language problems. However, only total cerebellar volume has been associated with language functioning, with contradicting results. The objective of this study was to ascertain whether total cerebellar structures or specific posterior lobular structures are associated with language ability of school-aged VPT children.
Methods
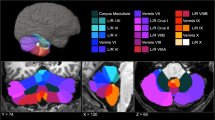
This is a prospective cohort study of 42 school-aged VPT children without major handicaps. Structural MRI was performed and the cerebellum segmentation pipeline was used for segmentation of separate lobules. Narrative retelling assessment was performed and language content and language structure scores were extracted. Linear regression analyses were used to associate language scores with whole gray matter (GM) cerebellar volume and right Crus I+II GM volume.
Results
Whole cerebellar GM volume was not significantly associated with language content nor with language structure; however, right Crus I+II GM volume was significantly associated with language content (β = 0.192 (CI = 0.033, 0.351), p = 0.020).
Conclusions
GM volume of Crus I+II appears to be associated with language functions in school-aged VPT children without major handicaps, while whole cerebellar volume is not. This study showed the importance of studying cerebellar lobules separately, rather than whole cerebellar volume only, in relation to VPT children’s language functions.
Impact
-
GM volume of Crus I+II is associated with semantic language functions in school-aged very preterm children without overt brain injury, whereas whole cerebellar volume is not.
-
This study showed the importance of studying cerebellar lobules separately, rather than whole cerebellar volume only, in relation to very preterm children’s language functions.
-
This study might impact future research in very preterm children. Lobular structures rather than whole cerebellar structures should be the region of interest in relation to language functions.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Nguyen, T. N. et al. Developmental trajectory of language from 2 to 13 years in children born very preterm. Pediatrics 141, e20172831 (2018).
van Noort-van der Spek, I. L., Franken, M. & Weisglas-Kuperus, N. Language functions in preterm-born children: a systematic review and meta-analysis. Pediatrics 129, 745–754 (2012).
Saigal, S. & Doyle, L. W. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 371, 261–269 (2008).
Elgen, S. K. et al. Mental health at 5 years among children born extremely preterm: a national population-based study. Eur. Child Adolesc. Psychiatry 21, 583–589 (2012).
Barre, N., Morgan, A., Doyle, L. W. & Anderson, P. J. Language abilities in children who were very preterm and/or very low birth weight: a meta-analysis. J. Pediatr. 158, 766.e1–774.e1 (2011).
Smith, J. M., DeThorne, L. S., Logan, J. A., Channell, R. W. & Petrill, S. A. Impact of prematurity on language skills at school age. J. Speech Lang. Hear. Res. 57, 901–916 (2014).
Stipdonk, L. W., Franken, M. J. P. & Dudink, J. Language outcome related to brain structures in school-aged preterm children: a systematic review. PLoS ONE 13, e0196607 (2018).
Kwon, S. H. et al. Functional magnetic resonance connectivity studies in infants born preterm: Suggestions of proximate and long-lasting changes in language organization. Dev. Med. Child Neurol. 58, 28–34 (2016).
de Kieviet, J. F., Zoetebier, L., van Elburg, R. M., Vermeulen, R. J. & Oosterlaan, J. Brain development of very preterm and very low-birthweight children in childhood and adolescence: a meta-analysis. Dev. Med. Child Neurol. 54, 313–323 (2012).
Arhan, E. et al. Regional brain volume reduction and cognitive outcomes in preterm children at low risk at 9 years of age. Childs Nerv. Syst. 33, 1317–1326 (2017).
Narberhaus, A. et al. Corpus callosum and prefrontal functions in adolescents with history of very preterm birth. Neuropsychologia 46, 111–116 (2008).
Northam, G. B. et al. Interhemispheric temporal lobe connectivity predicts language impairment in adolescents born preterm. Brain 135, 3781–3798 (2012).
Nosarti, C. et al. Corpus callosum size and very preterm birth: relationship to neuropsychological outcome. Brain 127, 2080–2089 (2004).
Murdoch, B. E. The cerebellum and language: historical perspective and review. Cortex 46, 858–868 (2010).
Volpe, J. J. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 8, 110–124 (2009).
Chang, C. H., Chang, F. M., Yu, C. H., Ko, H. C. & Chen, H. Y. Assessment of fetal cerebellar volume using three-dimensional ultrasound. Ultrasound Med. Biol. 26, 981–988 (2000).
Pieterman, K. et al. Cerebellar growth impairment characterizes school-aged children born preterm without perinatal brain lesions. AJNR Am. J. Neuroradiol. 39, 956–962 (2018).
Buckner, R. L. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron 80, 807–815 (2013).
Vias, C. & Dick, A. S. Cerebellar contributions to language in typical and atypical development: a review. Dev. Neuropsychol. 42, 404–421 (2017).
Bernard, J. A. et al. Resting state cortico-cerebellar functional connectivity networks: a comparison of anatomical and self-organizing map approaches. Front. Neuroanat. 6, 31 (2012).
Schmahmann, J. D. The cerebellum and cognition. Neurosci. Lett. 688, 62–75 (2019).
Schmahmann, J. D., Guell, X., Stoodley, C. J. & Halko, M. A. The theory and neuroscience of cerebellar cognition. Annu. Rev. Neurosci. 42, 337–364 (2019).
Sugihara, I. Crus I in the rodent cerebellum: its homology to Crus I and II in the primate cerebellum and its anatomical uniqueness among neighboring lobules. Cerebellum 17, 49–55 (2018).
Limperopoulos, C. et al. Injury to the premature cerebellum: outcome is related to remote cortical development. Cereb. Cortex 24, 728–736 (2014).
Stoodley, C. J. & Schmahmann, J. D. The cerebellum and language: evidence from patients with cerebellar degeneration. Brain Lang. 110, 149–153 (2009).
Moore, D. M., D’Mello, A. M., McGrath, L. M. & Stoodley, C. J. The developmental relationship between specific cognitive domains and grey matter in the cerebellum. Dev. Cogn. Neurosci. 24, 1–11 (2017).
Ranger, M. et al. Neonatal pain and infection relate to smaller cerebellum in very preterm children at school age. J. Pediatr. 167, 292.e1–298.e1 (2015).
Argyropoulos, G. P. D. et al. Neocerebellar Crus I abnormalities associated with a speech and language disorder due to a mutation in FOXP2. Cerebellum 18, 309–319 (2019).
Hodge, S. M. et al. Cerebellum, language, and cognition in autism and specific language impairment. J. Autism Dev. Disord. 40, 300–316 (2010).
D’Mello, A. M., Turkeltaub, P. E. & Stoodley, C. J. Cerebellar tDCS modulates neural circuits during semantic prediction: a combined tDCS-fMRI study. J. Neurosci. 37, 1604–1613 (2017).
Frings, M. et al. Cerebellar involvement in verb generation: an fMRI study. Neurosci. Lett. 409, 19–23 (2006).
Guediche, S., Holt, L. L., Laurent, P., Lim, S. J. & Fiez, J. A. Evidence for cerebellar contributions to adaptive plasticity in speech perception. Cereb. Cortex 25, 1867–1877 (2015).
Moberget, T., Gullesen, E. H., Andersson, S., Ivry, R. B. & Endestad, T. Generalized role for the cerebellum in encoding internal models: evidence from semantic processing. J. Neurosci. 34, 2871–2878 (2014).
Stoodley, C. J. & Schmahmann, J. D. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage 44, 489–501 (2009).
Gelinas, J. N., Fitzpatrick, K. P., Kim, H. C. & Bjornson, B. H. Cerebellar language mapping and cerebral language dominance in pediatric epilepsy surgery patients. Neuroimage Clin. 6, 296–306 (2014).
Matthews, L. G. et al. Longitudinal preterm cerebellar volume: perinatal and neurodevelopmental outcome associations. Cerebellum 17, 610–627 (2018).
Parker, J. et al. Cerebellar growth and behavioural & neuropsychological outcome in preterm adolescents. Brain 131, 1344–1351 (2008).
Brumbaugh, J. E. et al. Altered brain function, structure, and developmental trajectory in children born late preterm. Pediatr. Res. 80, 197–203 (2016).
Martinussen, M., Flanders, D. W., Fischl, B. & Busa, E. Segmental brain volumes and cognitive and perceptual correlates in 15-year-old adolescents with low birth weight. J. Pediatr. 155, 848.e1–853.e1 (2009).
Bruckert, L. et al. White matter plasticity in reading-related pathways differs in children born preterm and at term: a longitudinal analysis. Front. Hum. Neurosci. 13, 139 (2019).
Limperopoulos, C. et al. Cerebellar injury in the premature infant is associated with impaired growth of specific cerebral regions. Pediatr. Res. 68, 145–150 (2010).
Renfrew, C. E. The Bus Story: A Test of Continuous Speech (C Renfrew, North Place, Headington, 1969).
Boerma, T., Leseman, P., Timmermeister, M., Wijnen, F. & Blom, E. Narrative abilities of monolingual and bilingual children with and without language impairment: implications for clinical practice. Int. J. Lang. Commun. Disord. 51, 626–638 (2016).
Botting, N. Narrative as a tool for the assessment of linguitic and pragmatic impairments. Child Lang. Teach. Ther. 18, 1–21 (2002).
Stipdonk, L. W., Dudink, J., Reiss, I. K. & Franken, M. J. P. Does a narrative retelling task improve the assessment of language proficiency in school-aged children born very preterm? Clin. Linguist Phon. 34, 1112–1129 (2020).
Papile, L. A., Burstein, J., Burstein, R. & Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J. Pediatr. 92, 529–534 (1978).
de Vries, L. S., Eken, P. & Dubowitz, L. M. The spectrum of leukomalacia using cranial ultrasound. Behav. Brain Res. 49, 1–6 (1992).
Semel, E., Wiig, E. H. & Secord, W. A. Clinical Evaluation of Language Fundamentals-4 (Pearson, Amsterdam, 2010).
Backhausen, L. L. et al. Quality control of structural MRI images applied using FreeSurfer-a hands-on workflow to rate motion artifacts. Front. Neurosci. 10, 558 (2016).
Fischl, B. FreeSurfer. Neuroimage 62, 774–781 (2012).
Manjon, J. V. & Coupe, P. volBrain: an online MRI brain volumetry system. Front. Neuroinform. 10, 30 (2016).
Jansonius, K. et al. Renfrew Taalschalen Nederlandse Aanpassing (Garant, 2014).
MacWhinney, B. The CHILDES Project: Tools for Analysing Talk (Lawrence Erlbaum Associates, Mahwah, NJ, 2000).
Knecht, S. et al. Handedness and hemispheric language dominance in healthy humans. Brain 123, 2512–2518 (2000).
Bradshaw, A. R., Thompson, P. A., Wilson, A. C., Bishop, D. V. M. & Woodhead, Z. V. J. Measuring language lateralisation with different language tasks: a systematic review. PeerJ 5, e3929 (2017).
Aarnoudse-Moens, C. S., Weisglas-Kuperus, N., van Goudoever, J. B. & Oosterlaan, J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics 124, 717–728 (2009).
Acknowledgements
We thank all children and their parents for their participation in the study. This work was supported by Dr. C.J. Vaillantfonds, Stichting Mitialto, Stichting Coolsingel under Grant number 496, and Stichting Sophia Wetenschappelijk Onderzoek under Grant number S19-24.
Author information
Authors and Affiliations
Contributions
We confirm that each author has met the Pediatric Research authorship requirements. L.W.S., M.B., K.J.P., M.-C.J.P.F., J.v.R., and J.D. have substantially contributed to conception and design, acquisition of data, or analysis and interpretation of data. All authors have drafted the article or revised it critically for important intellectual content and have given final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
Parents of participants have given written informed consent for participation and publication.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Stipdonk, L.W., Boumeester, M., Pieterman, K.J. et al. Cerebellar volumes and language functions in school-aged children born very preterm. Pediatr Res 90, 853–860 (2021). https://doi.org/10.1038/s41390-020-01327-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-01327-z
This article is cited by
-
Early parenteral lipid intake supports cerebellar neurometabolism at term-age in preterm infants
Journal of Perinatology (2025)
-
Cerebellar Development and the Burden of Prematurity
The Cerebellum (2025)
-
Language performance and brain volumes, asymmetry, and cortical thickness in children born extremely preterm
Pediatric Research (2024)