Abstract
Background
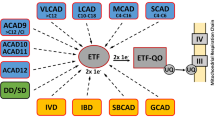
Medium-chain acyl-CoA dehydrogenase (MCAD) deficiency (MCADD) is the most frequent fatty acid oxidation (FAO) defect in humans. MCAD-deficient fibroblasts are more resistant to oxidative stress-induced cell death than other FAO defects and healthy controls.
Methods
Herein we investigate the antioxidant response and mitochondrial function in fibroblasts from MCAD-deficient patients (c.985 A>G/c.985 A>G) and healthy controls.
Results
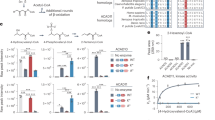
MCAD-deficient fibroblasts showed increased level of mitochondrial superoxide, while lipids were less oxidatively damaged, and higher amount of manganese superoxide dismutase were detected compared to healthy controls, showing forceful antioxidant system in MCADD. We showed increased maximal respiration and reserve capacity in MCAD-deficient fibroblasts compared to controls, indicating more capacity through the tricarboxylic acid (TCA) cycle and subsequently respiratory chain. This led us to study the pyruvate dehydrogenase complex (PDC), the key enzyme in the glycolysis releasing acetyl-CoA to the TCA cycle. MCAD-deficient fibroblasts displayed not only significantly increased PDC but also increased lipoylated PDC protein levels compared to healthy controls.
Conclusions
Based on these findings, we raise the interesting hypothesis that increased PDC-bound lipoic acid, synthesized from accumulated octanoic acid in MCADD, may affect the cellular antioxidant pool in MCADD.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Gregersen, N. et al. Mitochondrial fatty acid oxidation defects-remaining challenges. J. Inherit. Metab. Dis. 31, 643–657 (2008).
Gregersen, N., Bross, P. & Andresen, B. S. Genetic defects in fatty acid beta-oxidation and acyl-CoA dehydrogenases. Molecular pathogenesis and genotype-phenotype relationships. Eur. J. Biochem. 271, 470–482 (2004).
Tanaka, K. et al. A survey of the newborn populations in Belgium, Germany, Poland, Czech Republic, Hungary, Bulgaria, Spain, Turkey, and Japan for the G985 variant allele with haplotype analysis at the medium chain Acyl-CoA dehydrogenase gene locus: clinical and evolutionary consideration. Pediatr. Res. 41, 201–209 (1997).
Bross, P. et al. Effects of two mutations detected in medium chain acyl-CoA dehydrogenase (MCAD)-deficient patients on folding, oligomer assembly, and stability of MCAD enzyme. J. Biol. Chem. 270, 10284–10290 (1995).
Andresen, B. S. et al. MCAD deficiency in Denmark. Mol. Genet. Metab. 106, 175–188 (2012).
Derks, T. G. et al. Experimental evidence for protein oxidative damage and altered antioxidant defense in patients with medium-chain acyl-CoA dehydrogenase deficiency. J. Inherit. Metab. Dis. 37, 783–789 (2014).
Lim, S. C. et al. Loss of the mitochondrial fatty acid beta-oxidation protein medium-chain acyl-coenzyme A dehydrogenase disrupts oxidative phosphorylation protein complex stability and function. Sci. Rep. 8, 153 (2018).
Scaini, G. et al. Toxicity of octanoate and decanoate in rat peripheral tissues: evidence of bioenergetic dysfunction and oxidative damage induction in liver and skeletal muscle. Mol. Cell. Biochem. 361, 329–335 (2012).
Schuck, P. F. et al. Medium-chain fatty acids accumulating in MCAD deficiency elicit lipid and protein oxidative damage and decrease non-enzymatic antioxidant defenses in rat brain. Neurochem. Int. 54, 519–525 (2009).
Zolkipli, Z., Pedersen, C. B., Lamhonwah, A. M., Gregersen, N. & Tein, I. Vulnerability to oxidative stress in vitro in pathophysiology of mitochondrial short-chain acyl-CoA dehydrogenase deficiency: response to antioxidants. PLoS ONE 6, e17534 (2011).
Fernandez-Guerra, P. et al. Application of an image cytometry protocol for cellular and mitochondrial phenotyping on fibroblasts from patients with inherited disorders. JIMD Rep. 27, 17–26 (2016).
Zhou, Y. et al. Mitochondrial spare respiratory capacity is negatively correlated with nuclear reprogramming efficiency. Stem Cells Dev. 26, 166–176 (2017).
Birkler, R. I., Nochi, Z., Gregersen, N. & Palmfeldt, J. Selected reaction monitoring mass spectrometry for relative quantification of proteins involved in cellular life and death processes. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1035, 49–56 (2016).
Pedersen, C. B. et al. Antioxidant dysfunction: potential risk for neurotoxicity in ethylmalonic aciduria. J. Inherit. Metab. Dis. 33, 211–222 (2010).
Ostergaard, E. et al. Four novel PDHA1 mutations in pyruvate dehydrogenase deficiency. J. Inherit. Metab. Dis. 32(Suppl 1), S235–S239 (2009).
Drummen, G. P., Makkinje, M., Verkleij, A. J., Op den Kamp, J. A. & Post, J. A. Attenuation of lipid peroxidation by antioxidants in rat-1 fibroblasts: comparison of the lipid peroxidation reporter molecules cis-parinaric acid and C11-BODIPY(581/591) in a biological setting. Biochim. Biophys. Acta 1636, 136–150 (2004).
Perham, R. N. Swinging arms and swinging domains in multifunctional enzymes: catalytic machines for multistep reactions. Annu. Rev. Biochem. 69, 961–1004 (2000).
Mayr, J. A., Feichtinger, R. G., Tort, F., Ribes, A. & Sperl, W. Lipoic acid biosynthesis defects. J. Inherit. Metab. Dis. 37, 553–563 (2014).
Tort, F. et al. Mutations in the lipoyltransferase LIPT1 gene cause a fatal disease associated with a specific lipoylation defect of the 2-ketoacid dehydrogenase complexes. Hum. Mol. Genet. 23, 1907–1915 (2014).
Ayala, A., Munoz, M. F. & Arguelles, S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 360438 (2014).
Hill, B. G., Dranka, B. P., Zou, L., Chatham, J. C. & Darley-Usmar, V. M. Importance of the bioenergetic reserve capacity in response to cardiomyocyte stress induced by 4-hydroxynonenal. Biochem. J. 424, 99–107 (2009).
Nickens, K. P., Wikstrom, J. D., Shirihai, O. S., Patierno, S. R. & Ceryak, S. A bioenergetic profile of non-transformed fibroblasts uncovers a link between death-resistance and enhanced spare respiratory capacity. Mitochondrion 13, 662–667 (2013).
Liemburg-Apers, D. C., Willems, P. H., Koopman, W. J. & Grefte, S. Interactions between mitochondrial reactive oxygen species and cellular glucose metabolism. Arch. Toxicol. 89, 1209–1226 (2015).
Olsen, R. K., Cornelius, N. & Gregersen, N. Redox signalling and mitochondrial stress responses; lessons from inborn errors of metabolism. J. Inherit. Metab. Dis. 38, 703–719 (2015).
Rabinovitch, R. C. et al. AMPK maintains cellular metabolic homeostasis through regulation of mitochondrial reactive oxygen species. Cell Rep. 21, 1–9 (2017).
Heiss, E. H., Schachner, D., Zimmermann, K. & Dirsch, V. M. Glucose availability is a decisive factor for Nrf2-mediated gene expression. Redox Biol. 1, 359–365 (2013).
Ventura, F. V., Ruiter, J. P., IJ, L., de Almeida, I. T. & Wanders, R. J. Lactic acidosis in long-chain fatty acid beta-oxidation disorders. J. Inherit. Metab. Dis. 21, 645–654 (1998).
Iafolla, A. K., Thompson, R. J. Jr. & Roe, C. R. Medium-chain acyl-coenzyme A dehydrogenase deficiency: clinical course in 120 affected children. J. Pediatrics 124, 409–415 (1994).
Jackson, S. et al. Long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency. Pediatr. Res. 29, 406–411 (1991).
Tonin, A. M. et al. Long-chain 3-hydroxy fatty acids accumulating in LCHAD and MTP deficiencies induce oxidative stress in rat brain. Neurochem. Int. 56, 930–936 (2010).
Cornelius, N., Corydon, T. J., Gregersen, N. & Olsen, R. K. Cellular consequences of oxidative stress in riboflavin responsive multiple acyl-CoA dehydrogenation deficiency patient fibroblasts. Hum. Mol. Genet. 23, 4285–4301 (2014).
Olsen, R. K., Cornelius, N. & Gregersen, N. Genetic and cellular modifiers of oxidative stress: what can we learn from fatty acid oxidation defects? Mol. Genet. Metab. 110(Suppl), S31–S39 (2013).
Wang, B. et al. Effects of long-chain and medium-chain fatty acids on apoptosis and oxidative stress in human liver cells with steatosis. J. Food Sci. 81, H794–H800 (2016).
Estrada, D. E. et al. Stimulation of glucose uptake by the natural coenzyme alpha-lipoic acid/thioctic acid: participation of elements of the insulin signaling pathway. Diabetes 45, 1798–1804 (1996).
Khanna, S., Roy, S., Packer, L. & Sen, C. K. Cytokine-induced glucose uptake in skeletal muscle: redox regulation and the role of alpha-lipoic acid. Am. J. Physiol. 276, R1327–R1333 (1999).
Korotchkina, L. G., Yang, H., Tirosh, O., Packer, L. & Patel, M. S. Protection by thiols of the mitochondrial complexes from 4-hydroxy-2-nonenal. Free Radic. Biol. Med. 30, 992–999 (2001).
Cao, X., Zhu, L., Song, X., Hu, Z. & Cronan, J. E. Protein moonlighting elucidates the essential human pathway catalyzing lipoic acid assembly on its cognate enzymes. Proc. Natl Acad. Sci. USA 115, E7063–E7072 (2018).
Booker, S. J. Unraveling the pathway of lipoic acid biosynthesis. Chem. Biol. 11, 10–12 (2004).
Khanna, S. et al. Skeletal muscle and liver lipoyllysine content in response to exercise, training and dietary alpha-lipoic acid supplementation. Biochem. Mol. Biol. Int. 46, 297–306 (1998).
Acknowledgements
We thank the Faculty of Health Sciences at Aarhus University and the Danish Council for Independent Research (Grant Nos. #4004-00548 and #11-107331) for financial support.
Author information
Authors and Affiliations
Contributions
Z.N.: experimental design, performed the experiments, data analysis, and writing the first draft of the manuscript. R.I.D.B.: performed the mass spectrometric analysis. P.F.-G.: MitoSOX and lipid peroxidation data analysis, set up the Seahorse profile protocol, and revised the manuscript. J.H.: performed the acylcarnitine analysis and revised the manuscript. 5. F.W.: performed the pyruvate dehydrogenase activity measurement. T.J.C.: experimental design, report editing, and revising the manuscript. N.G. and R.K.J.O.: experimental design, report editing, data analysis, and critically revising the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nochi, Z., Birkler, R.I.D., Fernandez-Guerra, P. et al. Increased antioxidant response in medium-chain acyl-CoA dehydrogenase deficiency: does lipoic acid have a protective role?. Pediatr Res 88, 556–564 (2020). https://doi.org/10.1038/s41390-020-0801-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-0801-1
This article is cited by
-
Evidence that Oxidative Disbalance and Mitochondrial Dysfunction are Involved in the Pathophysiology of Fatty Acid Oxidation Disorders
Cellular and Molecular Neurobiology (2022)