Abstract
Background
The associations of renal, hepatic, and hematologic markers with metabolic risk (MR) have already been shown in adolescents. However, it is still controversial which marker best predicts metabolic changes in youth. The aim of this study was to verify the association of MR with alanine aminotransferase (ALT), aspartate aminotransferase (AST), uric acid, and hemoglobin (Hb) in adolescents.
Methods
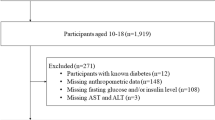
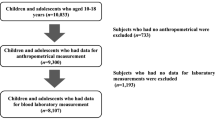
We evaluated 1713 Brazilian adolescents aged 10 to 17 years. MR was calculated using a continuous metabolic risk score, including the sum of Z-scores of waist circumference, systolic blood pressure, fasting glucose, high-density lipoproteins, triglycerides, and cardiorespiratory fitness. Cutoff points were set for MR prediction for five metabolic components (ALT, AST, AST/ALT ratio, uric acid, and Hb).
Results
MR was strongly associated with increased uric acid (odds ratio [OR]: 2.50; 95% confidence interval [CI]: 1.74–3.59), ALT (OR: 2.64; 95% CI: 1.63–4.27), and AST levels (OR: 2.53; 95% CI: 1.24–5.18). Uric acid was shown to be the best predictor for MR (sensitivity: 55.79%; specificity: 61.35%; area under the curve: 0.616).
Conclusion
Elevated hepatic, renal, and hematological markers were associated with MR in adolescents, especially ALT, AST, and uric acid levels.
Impact
-
Elevated hepatic, renal, and hematological markers were associated with metabolic risk in adolescents, especially ALT, AST, and uric acid levels.
-
It is still controversial which marker best predicts metabolic changes in adolescents. In addition, association of Hb with metabolic risk is under-studied in this population.
-
It is important to further investigate the relationship between elevated Hb and hepatic markers, since there are key aspects not addressed yet. Our results highlight the importance of creating public health policies aimed to child and adolescent population, to prevention of metabolic disorders from an early age.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Steinbeck, K. S., Lister, N. B., Gow, M. L. & Baur, L. A. Treatment of adolescent obesity. Nat. Rev. Endocrinol. 14, 331–344 (2018).
Agostinis-Sobrinho, C. A. et al. Muscular fitness and metabolic and inflammatory biomarkers in adolescents: results from LabMed Physical Activity Study. Scand. J. Med. Sci. Sports 27, 1873–1880 (2017).
Andersen, L. B. et al. A new approach to define and diagnose cardiometabolic disorder in children. J. Diabetes Res. 2015, 539835 (2015).
Reuter, C. P. et al. Cutoff points for continuous metabolic risk score in adolescents from southern Brazil. Am. J. Hum. Biol. 468, e23211 (2019).
Seo, J.-Y. & Kim, J. H. Validation of surrogate markers for metabolic syndrome and cardiometabolic risk factor clustering in children and adolescents: a nationwide population-based study. PLoS ONE 12, e0186050 (2017).
Welsh, J. A., Karpen, S. & Vos, M. B. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988–1994 to 2007–2010. J. Pediatr. 162, 496–500 (2013). e1.
Song, P. et al. Prevalence and correlates of suspected nonalcoholic fatty liver disease in Chinese children. Int. J. Environ. Res. Public Health 14, 465 (2017).
Fermin, C. R., Lee, A. M., Filipp, S. L., Gurka, M. J. & DeBoer, M. D. Serum Alanine aminotransferase trends and their relationship with obesity and metabolic syndrome in United States Adolescents, 1999–2014. Metab. Syndr. Relat. Disord. 15, 276–282 (2017).
Lee, K. & Yang, J. H. Which liver enzymes are better indicators of metabolic syndrome in adolescents: the Fifth Korea National Health and Nutrition Examination Survey, 2010. Metab. Syndr. Relat. Disord. 11, 229–235 (2013).
Lee, A. M., Gurka, M. J. & DeBoer, M. D. Correlation of metabolic syndrome severity with cardiovascular health markers in adolescents. Metabolism 69, 87–95 (2017).
Castillo-Durán, C., Sepúlveda, A. C., Espinoza, G. A., Rebollo, G. M. J. & Le Roy, O. C. Hyperuricaemia and metabolic syndrome in obese children and adolescents. Rev. Chil. Pediatr. 87, 18–23 (2016).
Chao, K.-C. et al. Hb and dyslipidaemia as predicting markers of serum alanine aminotransferase elevation in Chinese adolescents. Public Health Nutr. 19, 1067–1073 (2016).
PROESP. Sports Brazil Project https://www.ufrgs.br/proesp/ (2016).
Fernández, J. R., Redden, D. T., Pietrobelli, A. & Allison, D. B. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J. Pediatr. 145, 439–444 (2004).
Malachias, M. et al. 7th Brazilian Guideline of Arterial Hypertension: Chapter 10—Hypertension in children and adolescents. Arq. Bras. Cardiol. 107, 53–63 (2016).
Lira, A. R. F. & Oliveira, F. L. C. Escrivão MAMS, Colugnati FAB, Taddei JAAC. Hepatic steatosis in a school population of overweight and obese adolescents. J. Pediatr. (Rio J.) 86, 45–52 (2010).
Feig, D. I. & Johnson, R. J. Hyperuricemia in childhood primary hypertension. Hypertension 42, 247–252 (2003).
Tanner, J. Growth at Adolescence 2nd edn (Blackwell Scientific, Oxford, 1962).
Sun, H.-L., Pei, D., Lue, K.-H. & Chen, Y.-L. Uric acid levels can predict metabolic syndrome and hypertension in adolescents: a 10-year longitudinal study. PLoS ONE 10, e0143786 (2015).
Mohammadi, F. et al. Association of cardiometabolic risk factors and hepatic enzymes in a national sample of Iranian children and adolescents: the CASPIAN-III study. J. Pediatr. Gastroenterol. Nutr. 58, 463–468 (2014).
Wang, Z.-N. et al. The association between serum uric acid and metabolic syndrome among adolescents in northeast China. Int. J. Clin. Exp. Med. 8, 21122–21129 (2015).
Acknowledgements
This work was supported by the Coordination for the Improvement of Personnel in Higher Education – Brazil (CAPES) (finance code 001).
Author information
Authors and Affiliations
Contributions
S.d.S. participated in study conception and design, acquisition, and interpretation of data, as well drafted and revised the manuscript. C.P.R. contributed to study conception, acquisition, analysis and interpretation of data, and wrote and revised the manuscript. L.B.A., R.A.L., K.A.P., E.D.d.M., A.R.G., and S.I.R.F. assisted with study conception, interpretation of data, and wrote and revised the manuscript. J.D.P.R. conceptualized and designed the study, contributed to acquisition and interpretation of data, and wrote and revised the manuscript. All authors read and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Patient consent
All parents or guardians signed a free and informed consent form. In addition, schoolchildren aged 12 years and above signed a consent form.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Souza, S., Reuter, C.P., Andersen, L.B. et al. Metabolic risk associated with liver enzymes, uric acid, and hemoglobin in adolescents. Pediatr Res 88, 945–949 (2020). https://doi.org/10.1038/s41390-020-0832-7
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-0832-7