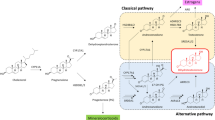
Abstract
BACKGROUND
We recently identified 35 women with polycystic ovarian syndrome (PCOS) who exhibited features of micronodular adrenocortical hyperplasia. Steroid hormone analysis can be more accurate using state-of-the-art ultra-performance convergence chromatography-tandem mass spectrometry (UPC2-MS/MS). We hypothesized that UPC2-MS/MS may be used to better define hormonally this distinct subgroup of patients with PCOS.
METHODS
Plasma from PCOS patients (n = 35) and healthy volunteers (HVs, n = 19) who all received dexamethasone testing was analyzed. Samples were grouped per dexamethasone responses and followed by UPC2-MS/MS analysis. When insufficient, samples were pooled from patients with similar responses to allow quantification over the low end of the assay.
RESULTS
The C11-oxy C19 (11β-hydroxyandrostenedione, 11keto-androstenedione, 11β-hydroxytestosterone, 11keto-testosterone):C19 (androstenedione, testosterone) steroid ratio was decreased by 1.75-fold in PCOS patients compared to HVs. Downstream steroid metabolites 11β-hydroxyandrosterone and 11keto-androsterone were also measurable. The C11-oxy C21 steroids, 11-hydroxyprogesterone and 11keto-dihydroprogesterone levels, were 1.2- and 1.7-fold higher in PCOS patients compared to HVs, respectively.
CONCLUSIONS
We hypothesized that UPC2-MS/MS may accurately quantify steroids, in vivo, and identify novel metabolites in a subgroup of patients with PCOS and adrenal abnormalities. Indeed, it appears that adrenal C11-oxy steroids have the potential of being used diagnostically to identify younger women and adolescents with PCOS who also have some evidence of micronodular adrenocortical hyperplasia.
Impact
-
Adrenal C11-oxy steroids may be clinically important in identifying young patients with PCOS and adrenal abnormalities.
-
The steroids presented in our manuscript have not yet been considered in the clinical setting so far, and we believe that this study could represent a first focused step towards the characterization of a distinct subgroup of women with PCOS who may in fact be treated differently than the average patient with PCOS.
-
This paper can change the understanding of PCOS as one disorder: it is in fact a heterogeneous condition. In addition, for the subgroup of patients with PCOS associated with adrenocortical dysfunction, our paper provides novel hormonal markers that can be used diagnostically. Finally, the paper also adds to the basic pathophysiological understanding of adrenocortical-ovarian interactions in steroidogenesis of young women and adolescent girls with PCOS.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Rosenfield, R. L. & Ehrmann, D. A. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr. Rev. 37, 467–520 (2016).
Gourgari, E. et al. Bilateral adrenal hyperplasia as a possible mechanism for hyperandrogenism in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 101, 3353–3360 (2016).
Crutchfield, C. A. et al. Comprehensive analysis of LC/MS data using pseudocolor plots. J. Am. Soc. Mass Spectrom. 24, 230–237 (2013).
Azziz, R., Black, V. Y., Knochenhauer, E. S., Hines, G. A. & Boots, L. R. Ovulation after glucocorticoid suppression of adrenal androgens in the polycystic ovary syndrome is not predicted by the basal dehydroepiandrosterone sulfate level. J. Clin. Endocrinol. Metab. 84, 946–950 (1999).
Rege, J. et al. Liquid chromatography-tandem mass spectrometry analysis of human adrenal vein 19-carbon steroids before and after ACTH stimulation. J. Clin. Endocrinol. Metab. 98, 1182–1188 (2013).
Maas, K. H. et al. Androgen responses to adrenocorticotropic hormone infusion among individual women with polycystic ovary syndrome. Fertil. Steril. 106, 1252–1257 (2016).
Yildiz, B. O. & Azziz, R. The adrenal and polycystic ovary syndrome. Rev. Endocr. Metab. Disord. 8, 331–342 (2007).
Kamrath, C., Wettstaedt, L., Boettcher, C., Hartmann, M. F. & Wudy, S. A. Androgen excess is due to elevated 11-oxygenated androgens in treated children with congenital adrenal hyperplasia. J. Steroid Biochem. Mol. Biol. 178, 221–228 (2018).
O’Reilly, M. W. et al. 11-Oxygenated C19 steroids are the predominant androgens in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 102, 840–848 (2017).
Davis, S. R., Turcu, A. F., Robinson, P. J. & Bell, R. J. Exogenous testosterone does not influence 11-oxygenated C19 steroid concentrations in healthy postmenopausal women. J. Endocr. Soc. 3, 670–677 (2019).
Turcu, A. F. & Auchus, R. J. Clinical significance of 11-oxygenated androgens. Curr. Opin. Endocrinol. Diabetes Obes. 24, 252–259 (2017).
du Toit, T. et al. Profiling adrenal 11beta-hydroxyandrostenedione metabolites in prostate cancer cells, tissue and plasma: UPC(2)-MS/MS quantification of 11beta-hydroxytestosterone, 11keto-testosterone and 11keto-dihydrotestosterone. J. Steroid Biochem. Mol. Biol. 166, 54–67 (2017).
du Toit, T. & Swart, A. C. Inefficient UGT-conjugation of adrenal 11beta-hydroxyandrostenedione metabolites highlights C11-oxy C19 steroids as the predominant androgens in prostate cancer. Mol. Cell. Endocrinol. 461, 265–276 (2018).
Turcu, A. F. et al. Adrenal-derived 11-oxygenated 19-carbon steroids are the dominant androgens in classic 21-hydroxylase deficiency. Eur. J. Endocrinol. 174, 601–609 (2016).
du Toit, T., Stander, M. A. & Swart, A. C. A high-throughput UPC(2)-MS/MS method for the separation and quantification of C19 and C21 steroids and their C11-oxy steroid metabolites in the classical, alternative, backdoor and 11OHA4 steroid pathways. J. Chromatogr. B 1080, 71–81 (2018).
Bloem, L. M. et al. Advances in the analytical methodologies: profiling steroids in familiar pathways—challenging dogmas. J. Steroid Biochem. Mol. Biol. 153, 80–92 (2015).
Choi, M. H., Skipper, P. L., Wishnok, J. S. & Tannenbaum, S. R. Characterization of testosterone 11 beta-hydroxylation catalyzed by human liver microsomal cytochromes P450. Drug Metab. Dispos. 33, 714–718 (2005).
Swart, P., Swart, A. C., Waterman, M. R., Estabrook, R. W. & Mason, J. I. Progesterone 16 alpha-hydroxylase activity is catalyzed by human cytochrome P450 17 alpha-hydroxylase. J. Clin. Endocrinol. Metab. 77, 98–102 (1993).
Swart, A. C., Kolar, N. W., Lombard, N., Mason, J. I. & Swart, P. Baboon cytochrome P450 17alpha-hydroxylase/17,20-lyase (CYP17). Eur. J. Biochem. 269, 5608–5616 (2002).
van Rooyen, D. et al. The metabolic fate and receptor interaction of 16alpha-hydroxyprogesterone and its 5alpha-reduced metabolite, 16alpha-hydroxy-dihydroprogesterone. Mol. Cell Endocrinol. 441, 86–98 (2017).
van Rooyen, D., Gent, R., Barnard, L. & Swart, A. C. The in vitro metabolism of 11beta-hydroxyprogesterone and 11-ketoprogesterone to 11-ketodihydrotestosterone in the backdoor pathway. J. Steroid Biochem. Mol. Biol. 178, 203–212 (2018).
Tonetto-Fernandes, V. et al. Serum 21-deoxycortisol, 17-hydroxyprogesterone, and 11-deoxycortisol in classic congenital adrenal hyperplasia: clinical and hormonal correlations and identification of patients with 11beta-hydroxylase deficiency among a large group with alleged 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 91, 2179–2184 (2006).
Turcu, A. F. et al. Profiles of 21-carbon steroids in 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 100, 2283–2290 (2015).
Rodin, A., Thakkar, H., Taylor, N. & Clayton, R. Hyperandrogenism in polycystic ovary syndrome. Evidence of dysregulation of 11 beta-hydroxysteroid dehydrogenase. N. Engl. J. Med. 330, 460–465 (1994).
Vassiliadi, D. A. et al. Increased 5 alpha-reductase activity and adrenocortical drive in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 94, 3558–3566 (2009).
Barnard, L., Gent, R., van Rooyen, D. & Swart, A. C. Adrenal C11-oxy C21 steroids contribute to the C11-oxy C19 steroid pool via the backdoor pathway in the biosynthesis and metabolism of 21-deoxycortisol and 21-deoxycortisone. J. Steroid Biochem. Mol. Biol. 174, 86–95 (2017).
Gent, R., du Toit, T., Bloem, L. M. & Swart, A. C. The 11beta-hydroxysteroid dehydrogenase isoforms: pivotal catalytic activities yield potent C11-oxy C19 steroids with 11betaHSD2 favouring 11-ketotestosterone, 11-ketoandrostenedione and 11-ketoprogesterone biosynthesis. J. Steroid Biochem. Mol. Biol. 189, 116–126 (2019).
Acknowledgements
We thank Dr. Maya Lodish for assisting with the recruitment of patients and running the clinical protocol before its conclusion. We also thank Drs. Al Yergey (now deceased) and Peter Backlund of the NICHD Proteomics Core, who encouraged us to study novel steroid biomarkers in our patients and provided the first data that pointed to new steroid molecules in our patients. Many thanks to Drs. Elena Belyavskaya and Charalampos Lyssikatos for the collection, and Mrs. Maria de la Luz (Lucy) Sierra for handling successfully all the complexities of the sampling of this (and other) protocols. Finally, we are indebted to our HVs, our patients and their families who participated in this study, as well as the nursing and technical staff of the 1 and 5NW wards of the NIH-CRC; without them this study could never have been completed. This work was supported by National Research Foundation (IFR170125217588, CSUR160414162143); L’Oréal-UNESCO For Women in Science Sub-Saharan Africa Regional Fellowships, NIH-CRC Bench-to-Bedside Program and the NIH’s Office of Women’s Health; and in part by the NICHD Intramural Research Program, Bethesda, MD, USA.
Author information
Authors and Affiliations
Contributions
Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data (A.C.S., T. du T., E.G., M.K., M.K., F.R.F., C.A.S.); drafting the article or revising it critically for important intellectual content (A.C.S., E.G., C.A.S.); final approval of the version to be published (A.C.S., C.A.S.).
Corresponding author
Ethics declarations
Competing interests
C.A.S. holds patent on the PRKAR1A, PDE11A, and GPR101 genes and/or their function and his laboratory has received research funding from Pfizer Inc. F.R.F. holds patent on the GPR101 gene and/or its function. The other authors have nothing to disclose.
Patient consent
Informed consent was obtained from all HVs and adult patients; the patients’ parents for all pediatric patients and older children signed an assent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Swart, A.C., du Toit, T., Gourgari, E. et al. Steroid hormone analysis of adolescents and young women with polycystic ovarian syndrome and adrenocortical dysfunction using UPC2-MS/MS. Pediatr Res 89, 118–126 (2021). https://doi.org/10.1038/s41390-020-0870-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-0870-1