Abstract
Background
Poorly performing diagnostic tests can impact patient safety. Clinical investigations must have good precision and diagnostic accuracy before widespread use in clinical practice. Transient elastography (TE) measures liver stiffness, a surrogate marker of liver fibrosis in adults and children. Studies to evaluate its repeatability and reproducibility (precision) in children are limited. Our aim was to determine (i) the normal range of TE measurements and (ii) the repeatability and reproducibility of TE in healthy children.
Methods
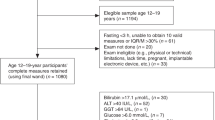
TE was performed in 257 healthy children, of whom 235 (91%, mean age 11.7 years, standard deviation (SD) 2.51, 107 were males (45.5%)) had two valid TE measurements performed, at least 24 h apart, by two operators under similar circumstances. High-quality TE images were obtained for each examination.
Results
The normal range of TE was 2.88–6.52 kPa. The mean difference between paired measurements was 0.044 (SD 0.4). The 95% limits of agreement ranged from −0.8 to +0.76 kPa for repeat measurements. There was a difference of >1 kPa between measurements in 61/235 (25.9%) children. The lack of precision was similar across all age groups.
Conclusions
This study demonstrates that TE does not have acceptable precision in healthy children, because random measurement variation results in the lack of agreement between paired measurements.
Impact
-
The precision and diagnostic accuracy of a new technology must be determined before it is deployed in children in order to ensure that appropriate clinical decisions are made, and healthcare resources are not wasted.
-
TE is widely used to diagnose liver disease in children without adequate evaluation of the precision (repeatability) of TE either in healthy children or children with liver disease.
-
This study demonstrates that TE does not have adequate precision in children.
-
This study was performed in accordance with methods previously published for children. Refinements to the test protocol, such as duration of fasting or probe size, will have to be evaluated for their impact on precision and accuracy before the test is deployed in research studies or clinical practice.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Friedrich-Rust, M., Poynard, T. & Castera, L. Critical comparison of elastography methods to assess chronic liver disease. Nat. Rev. Gastroenterol. Hepatol. 13, 402–411 (2016).
Tsochatzis, E. A. et al. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J. Hepatol. 54, 650–659 (2011).
Vuppalanchi, R. & Sanyal, A. J. Myths and mysteries about staging hepatic fibrosis by fibroscan. Clin. Gastroenterol. Hepatol. 13, 780–782 (2015).
Fraquelli, M. et al. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut 56, 968–973 (2007).
Nascimbeni, F. et al. Significant variations in elastometry measurements made within short-term in patients with chronic liver diseases. Clin. Gastroenterol. Hepatol. 13, 763–771 e1 (2015). -6.
Vignier, N. et al. Reproducibility of liver stiffness measurements in hepatitis C virus (HCV)-infected patients in Egypt. J. Viral Hepat. 18, e358–e365 (2011).
Roca, B., Resino, E., Torres, V., Herrero, E. & Penades, M. Interobserver discrepancy in liver fibrosis using transient elastography. J. Viral Hepat. 19, 711–715 (2012).
Millonig, G. et al. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology 48, 1718–1723 (2008).
Bland, J. M. & Altman, D. G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 8, 135–160 (1999).
Engelmann, G. et al. Feasibility study and control values of transient elastography in healthy children. Eur. J. Pediatr. 171, 353–360 (2012).
Goldschmidt, I. et al. Application and limitations of transient liver elastography in children. J. Pediatr. Gastroenterol. Nutr. 57, 109–113 (2013).
Lewindon, P. J. et al. Transient liver elastography in unsedated control children: impact of age and intercurrent illness. J. Paediatr. Child Health 52, 637–642 (2016).
Fitzpatrick, E., Quaglia, A., Vimalesvaran, S., Basso, M. S. & Dhawan, A. Transient elastography is a useful noninvasive tool for the evaluation of fibrosis in paediatric chronic liver disease. J. Pediatr. Gastroenterol. Nutr. 56, 72–76 (2013).
Lee, C. K. et al. Serum biomarkers and transient elastography as predictors of advanced liver fibrosis in a United States cohort: the Boston children’s hospital experience. J. Pediatr. 163, 1058–1064 e2 (2013).
Lee, C. K. et al. Validation of transient elastography cut points to assess advanced liver fibrosis in children and young adults: The Boston Children’s Hospital Experience. J. Pediatr. 198, 84–89 e2 (2018).
Malbrunot-Wagner, A. C. et al. Transient elastography and portal hypertension in pediatric patients with cystic fibrosis. J. Cyst. Fibros. 10, 338–342 (2011).
Menten, R. et al. Transient elastography in patients with cystic fibrosis. Pediatr. Radiol. 40, 1231–1235 (2010).
Friedrich-Rust, M. et al. Non-invasive measurement of liver and pancreas fibrosis in patients with cystic fibrosis. J. Cyst. Fibros. 12, 431–439 (2013).
Nobili, V. et al. Accuracy and reproducibility of transient elastography for the diagnosis of fibrosis in pediatric nonalcoholic steatohepatitis. Hepatology 48, 442–448 (2008).
Witters, P. et al. Non-invasive liver elastography (Fibroscan) for detection of cystic fibrosis-associated liver disease. J. Cyst. Fibros. 8, 392–399 (2009).
Behairy Bel, S., Sira, M. M., Zalata, K. R., Salama el, S. E. & Abd-Allah, M. A. Transient elastography compared to liver biopsy and morphometry for predicting fibrosis in pediatric chronic liver disease: Does etiology matter? World J. Gastroenterol. 22, 4238–4249 (2016).
Rowland, M. Liver disease in cystic fibrosis—a clinical challange in the era of improved outcomes. J. Cyst. Fibros. 18(Suppl. 1), S143–S144 (2019).
HSE. UK-WHO-Ireland Growth Charts https://www.hse.ie/eng/health/child/growthmonitoring/ (2013).
Tang, A., Cloutier, G., Szeverenyi, N. M. & Sirlin, C. B. Ultrasound elastography and MR elastography for assessing liver fibrosis: Part 1, principles and techniques. Am. J. Roentgenol. 205, 22–32 (2015).
Moore, S. C., Munnings, C. R., Brettle, D. S. & Evans, J. A. Assessment of ultrasound monitor image display performance. Ultrasound Med. Biol. 37, 971–979 (2011).
Boursier, J. et al. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology 57, 1182–1191 (2013).
Altman, D. G. Assessing new methods of clinical measurement. Br. J. Gen. Pract. 59, 399–400 (2009).
McAlinden, C., Khadka, J. & Pesudovs, K. Precision (repeatability and reproducibility) studies and sample-size calculation. J. Cataract Refract. Surg. 41, 2598–2604 (2015).
Barnhart, H. X., Lokhnygina, Y., Kosinski, A. S. & Haber, M. Comparison of concordance correlation coefficient and coefficient of individual agreement in assessing agreement. J. Biopharm. Stat. 17, 721–738 (2007).
McGrath, T. A. et al. Overinterpretation of research findings: evidence of “Spin” in systematic reviews of diagnostic accuracy studies. Clin. Chem. 63, 1353–1362. (2017).
Lord, S. J., Staub, L. P., Bossuyt, P. M. & Irwig, L. M. Target practice: choosing target conditions for test accuracy studies that are relevant to clinical practice. BMJ 343, d4684 (2011).
Harris, E. F. & Smith, R. N. Accounting for measurement error: a critical but often overlooked process. Arch. Oral. Biol. 54(Suppl. 1), S107–S117 (2009).
Anvari, A., Halpern, E. F. & Samir, A. E. Statistics 101 for radiologists. RadioGraphics 35, 1789–1801 (2015).
Watson, P. F. & Petrie, A. Method agreement analysis: a review of correct methodology. Theriogenology 73, 1167–1179 (2010).
Sandrin, L. et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med. Biol. 29, 1705–1713 (2003).
Kim, S. et al. Points to be considered when applying FibroScan S probe in children with biliary atresia. J. Pediatr. Gastroenterol. Nutr. 59, 624–628 (2014).
Hwang, J. Y. et al. Diagnostic performance of transient elastography for liver fibrosis in children: a systematic review and meta-analysis. Am. J. Roentgenol. 211, W257–W266. (2018).
Arena, U. et al. Liver stiffness is influenced by a standardized meal in patients with chronic hepatitis C virus at different stages of fibrotic evolution. Hepatology 58, 65–72 (2013).
Berzigotti, A. et al. Effect of meal ingestion on liver stiffness in patients with cirrhosis and portal hypertension. PLoS ONE 8, e58742 (2013).
Bossuyt, P. M. et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 351, h5527 (2015).
Acknowledgements
We thank all those who participated in this study, in particular our healthy volunteers without whom this study would not have been possible. This research was funded by The Health Research Board (HRA-PHS-662). The Health Research Board had no role in the study. There was no industry support for this study. The corresponding author had full access to all the data and has final responsibility for the decision to submit for publication.
Cystic Fibrosis Liver Disease Research Group
B. Casserly11, D. Cox3, A. Das12, C. Gallagher7, P. Greally13, C. Gunaratnam14, F. Healy15, M. Herzig16, S. Javadpour3, G. Lean13, N. G. McElvaney14, E. McKone7, D. Mullane17, M. Ni Chroinin17, M. O’ Mahony16, M. O’ Neil18, B. Plant17, M. Rogan12 and C. Short17
Author information
Authors and Affiliations
Consortia
Contributions
Study concept: M.R., B.B. and P.A.McC. Study design: M.R. and L.E.D. Acquisition of data: A.McG., J.D. and L.R. Data analysis: A.McG., L.E.D. and M.R. Data interpretation: B.L., L.R., E.F., B.B., J.D., P.McN., A.McG. and M.R. First draft manuscript: M.R. and A.McG. Critical revision of manuscript: B.B., B.D., A.McG. and M.R. Final appraisal of manuscript: B.B., B.D., P.A.McC., B.L., E.F., L.R., A.B., J.D., L.C., P.McN., Cystic Fibrosis Registry of Ireland and Cystic Fibrosis Liver Disease Research Group
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was approved by the Ethics Committees of Our Lady’s Children’s Hospital Crumlin (for both healthy volunteers and children with cystic fibrosis) and by the National Children’s Hospital Tallaght, Children’s University Hospital and St Vincent’s University Hospital Dublin for participants with cystic fibrosis. Written informed consent was obtained from parents or guardians of children under 18 years of age for their child’s participation in the study with assent from the children. Participants over 18 years of age provided written informed consent for the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rowland, M., McGee, A., Broderick, A. et al. Repeatability of transient elastography in children. Pediatr Res 88, 587–592 (2020). https://doi.org/10.1038/s41390-020-0916-4
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-0916-4
This article is cited by
-
Transient elastography lacks precision in children
Pediatric Research (2022)
-
Rigorous and consistent evaluation of diagnostic tests in children: another unmet need
Pediatric Research (2020)