Abstract
Background
Bronchopulmonary dysplasia remains one of the most common complications of prematurity, despite significant improvements in perinatal care. Functional modeling of human lung development and disease, like BPD, is limited by our ability to access the lung and to maintain relevant progenitor cell populations in culture.
Methods
We supplemented Rho/SMAD signaling inhibition with mTOR inhibition to generate epithelial basal cell-like cell lines from tracheal aspirates of neonates.
Results
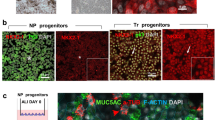
Single-cell RNA-sequencing confirmed the presence of epithelial cells in tracheal aspirates obtained from intubated neonates. Using Rho/SMAD/mTOR triple signaling inhibition, neonatal tracheal aspirate-derived (nTAD) basal cell-like cells can be expanded long term and retain the ability to differentiate into pseudostratified airway epithelium.
Conclusions
Our data demonstrate that neonatal tracheal aspirate-derived epithelial cells can provide a novel ex vivo human cellular model to study neonatal lung development and disease.
Impact
-
Airway epithelial basal cell-like cell lines were derived from human neonatal tracheal aspirates.
-
mTOR inhibition significantly extends in vitro proliferation of neonatal tracheal aspirate-derived basal cell-like cells (nTAD BCCs).
-
nTAD BCCs can be differentiated into functional airway epithelium.
-
nTAD BCCs provide a novel model to investigate perinatal lung development and diseases.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Jobe, A. H. Mechanisms of lung injury and bronchopulmonary dysplasia. Am. J. Perinatol. 33, 1076–1078 (2016).
McEvoy, C. T. et al. Bronchopulmonary dysplasia: NHLBI Workshop on the Primary Prevention of Chronic Lung Diseases. Ann. Am. Thorac. Soc. 11(Suppl 3), S146–S153 (2014).
Kotecha, S., Wangoo, A., Silverman, M. & Shaw, R. J. Increase in the concentration of transforming growth factor beta-1 in bronchoalveolar lavage fluid before development of chronic lung disease of prematurity. J. Pediatr. 128, 464–469 (1996).
Thompson, A. & Bhandari, V. Pulmonary biomarkers of bronchopulmonary dysplasia. Biomark. Insights 3, 361–373 (2008).
Hennrick, K. T. et al. Lung cells from neonates show a mesenchymal stem cell phenotype. Am. J. Respir. Crit. Care Med. 175, 1158–1164 (2007).
Spadafora, R. et al. Lung-resident mesenchymal stromal cells reveal transcriptional dynamics of lung development in preterm infants. Am. J. Respir. Crit. Care Med. 198, 961–964 (2018).
Wang, Q. et al. A novel in vitro model of primary human pediatric lung epithelial cells. Pediatr. Res. 87, 551–517 (2019).
Mou, H. et al. Dual SMAD signaling inhibition enables long-term expansion of diverse epithelial basal cells. Cell Stem Cell 19, 217–231 (2016).
Peters-Hall, J. R. et al. Long-term culture and cloning of primary human bronchial basal cells that maintain multipotent differentiation capacity and CFTR channel function. Am. J. Physiol. Lung Cell Mol. Physiol. 315, L313–L327 (2018).
Zhang, C. et al. Long-term in vitro expansion of epithelial stem cells enabled by pharmacological inhibition of PAK1-ROCK-Myosin II and TGF-beta signaling. Cell Rep. 25, 598–610 e595 (2018).
Langhofer, M., Hopkinson, S. B. & Jones, J. C. The matrix secreted by 804G cells contains laminin-related components that participate in hemidesmosome assembly in vitro. J. Cell Sci. 105(Pt 3), 753–764 (1993).
Levardon, H., Yonker, L. M., Hurley B. P. & Mou, H. Expansion of airway basal cells and generation of polarized epithelium. Bio. Protoc. 8, pii e2877 (2018).
Gierahn, T. M. et al. Seq-Well: portable, low-cost RNA sequencing of single cells at high throughput. Nat. Methods 14, 395–398 (2017).
Macosko, E. Z. et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161, 1202–1214 (2015).
Satija, R., Farrell, J. A., Gennert, D., Schier, A. F. & Regev, A. Spatial reconstruction of single-cell gene expression data. Nat. Biotechnol. 33, 495–502 (2015).
Du, Y. et al. Lung Gene Expression Analysis (LGEA): an integrative web portal for comprehensive gene expression data analysis in lung development. Thorax 72, 481–484 (2017).
Anders, S., Pyl, P. T. & Huber, W. HTSeq-a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Liberzon, A. et al. Molecular signatures database (MSigDB) 3.0. Bioinformatics 27, 1739–1740 (2011).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550 (2005).
Szklarczyk, D. et al. STRING v11: protein−protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 47, D607–D613 (2019).
Consortium, G. T. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 45, 580–585 (2013).
Jain, A. & Tuteja, G. TissueEnrich: tissue-specific gene enrichment analysis. Bioinformatics 35, 1966–1967 (2019).
Montoro, D. T. et al. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature 560, 319–324 (2018).
Plasschaert, L. W. et al. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature 560, 377–381 (2018).
Gidfar, S. et al. Rapamycin prolongs the survival of corneal epithelial cells in culture. Sci. Rep. 7, 40308 (2017).
Haller, S. et al. mTORC1 activation during repeated regeneration impairs somatic stem cell maintenance. Cell Stem Cell 21, 806–818 e805 (2017).
Iglesias-Bartolome, R. et al. mTOR inhibition prevents epithelial stem cell senescence and protects from radiation-induced mucositis. Cell Stem Cell 11, 401–414 (2012).
Feldman, M. E. et al. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 7, e38 (2009).
Guertin, D. A. & Sabatini, D. M. The pharmacology of mTOR inhibition. Sci. Signal. 2, pe24 (2009).
Saxton, R. A. & Sabatini, D. M. mTOR signaling in growth, metabolism, and disease. Cell 169, 361–371 (2017).
Kanehisa, M. & Goto, S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30 (2000).
Serra, V. et al. RSK3/4 mediate resistance to PI3K pathway inhibitors in breast cancer. J. Clin. Invest. 123, 2551–2563 (2013).
Liu, X. et al. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am. J. Pathol. 180, 599–607 (2012).
Rheinwald, J. G. & Green, H. Formation of a keratinizing epithelium in culture by a cloned cell line derived from a teratoma. Cell 6, 317–330 (1975).
Wang, X. et al. Cloning and variation of ground state intestinal stem cells. Nature 522, 173–178 (2015).
Hayflick, L. & Moorhead, P. S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 25, 585–621 (1961).
Xiang, S. et al. Rapamycin inhibits epithelial-to-mesenchymal transition of peritoneal mesothelium cells through regulation of Rho GTPases. FEBS J. 283, 2309–2325 (2016).
Behjati, S., Lindsay, S., Teichmann, S. A. & Haniffa, M. Mapping human development at single-cell resolution. Development 145, pii: dev152561 (2018).
Acknowledgements
This work was supported by the Department of Pediatrics, Massachusetts General Hospital. This work was also partly supported by the Harvard Stem Cell Institute (H.M.) and the Charles H. Hood Foundation (H.M.).
Author information
Authors and Affiliations
Contributions
J.L.: Substantial contributions to conception and design, acquisition of data, and analysis and interpretation of data; drafting the article or revising it critically for important intellectual content. X.Z.: Substantial contributions to conception and design, acquisition of data, and analysis and interpretation of data. J.E.S., L.X., T.G., C.Z., M.W., B.C.C.: Acquisition of data, and analysis and interpretation of data. J.C.L., H.L.: Analysis and interpretation of data. S.H.: Acquisition of data. H.M.: Substantial contributions to conception and design, analysis and interpretation of data; revising the article critically for important intellectual content. P.H.L.: Substantial contributions to conception and design, analysis and interpretation of data; revising the article critically for important intellectual content, and final approval of the version to be published.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Patient consent
Patient samples were collected using informed consent forms approved by the Institutional Review Board of Partners HealthCare.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Lu, J., Zhu, X., Shui, J.E. et al. Rho/SMAD/mTOR triple inhibition enables long-term expansion of human neonatal tracheal aspirate-derived airway basal cell-like cells. Pediatr Res 89, 502–509 (2021). https://doi.org/10.1038/s41390-020-0925-3
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-0925-3
This article is cited by
-
Perinatal origins of bronchopulmonary dysplasia—deciphering normal and impaired lung development cell by cell
Molecular and Cellular Pediatrics (2023)
-
CircRNA, lncRNA, and mRNA profiles of umbilical cord blood exosomes from preterm newborns showing bronchopulmonary dysplasia
European Journal of Pediatrics (2022)
-
Long-term differentiating primary human airway epithelial cell cultures: how far are we?
Cell Communication and Signaling (2021)
-
Prematurity alters the progenitor cell program of the upper respiratory tract of neonates
Scientific Reports (2021)