Abstract
Background
Unconjugated hyperbilirubinemia, a feature of neonatal jaundice or Crigler–Najjar syndrome, can lead to neurotoxicity and even death. We previously demonstrated that unconjugated bilirubin (UCB) can be eliminated via transintestinal excretion in Gunn rats, a model of unconjugated hyperbilirubinemia, and that this is stimulated by enhancing fecal fatty acid excretion. Since transintestinal excretion also occurs for cholesterol (TICE), we hypothesized that increasing fecal cholesterol excretion and/or TICE could also enhance fecal UCB disposal and subsequently lower plasma UCB concentrations.
Methods
To determine whether increasing fecal cholesterol excretion could ameliorate unconjugated hyperbilirubinemia, we treated hyperbilirubinemic Gunn rats with ezetimibe (EZE), an intestinal cholesterol absorption inhibitor, and/or a liver X receptor (LXR) and farnesoid X receptor (FXR) agonist (T0901317 (T09) and obeticholic acid (OCA), respectively), known to stimulate TICE.
Results
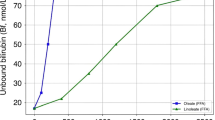
We found that EZE treatment alone or in combination with T09 or OCA increased fecal cholesterol disposal but did not lower plasma UCB levels.
Conclusions
These findings do not support a link between the regulation of transintestinal excretion of cholesterol and bilirubin. Furthermore, induction of fecal cholesterol excretion is not a potential therapy for unconjugated hyperbilirubinemia.
Impact
-
Increasing fecal cholesterol excretion is not effective to treat unconjugated hyperbilirubinemia.
-
This is the first time a potential relation between transintestinal excretion of cholesterol and unconjugated bilirubin is investigated.
-
Transintestinal excretion of cholesterol and unconjugated bilirubin do not seem to be quantitatively linked.
-
Unlike intestinal fatty acids, cholesterol cannot “capture” unconjugated bilirubin to increase its excretion.
-
These results add to our understanding of ways to improve and factors regulating unconjugated bilirubin disposal in hyperbilirubinemic conditions.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ostrow, J. D., Pascolo, L., Brites, D. & Tiribelli, C. Molecular basis of bilirubin-induced neurotoxicity. Trends Mol. Med. 10, 65–70 (2004).
McDonnell, W. M., Hitomi, E. & Askari, F. K. Identification of bilirubin UDP-GTs in the human alimentary tract in accordance with the gut as a putative metabolic organ. Biochem. Pharmacol. 51, 483–488 (1996).
Arias, I. M., Johnson, L. & Wolfson, S. Biliary excretion of injected conjugated and unconjugated bilirubin by normal and Gunn rats. Am. J. Physiol. 200, 1091–1094 (1961).
Kotal, P. et al. Intestinal excretion of unconjugated bilirubin in man and rats with inherited unconjugated hyperbilirubinemia. Pediatr. Res. 42, 195–200 (1997).
Nishioka, T. et al. Orlistat treatment increases fecal bilirubin excretion and decreases plasma bilirubin concentrations in hyperbilirubinemic Gunn rats. J. Pediatr. 143, 327–334 (2003).
Cuperus, F. J. C. et al. Effective treatment of unconjugated hyperbilirubinemia with oral bile salts in Gunn rats. Gastroenterology 136, 673.e1–682.e1 (2009).
Kruit, J. K. et al. Increased fecal neutral sterol loss upon liver X receptor activation is independent of biliary sterol secretion in mice. Gastroenterology 128, 147–156 (2005).
van der Veen, J. N. et al. Activation of the liver X receptor stimulates trans-intestinal excretion of plasma cholesterol. J. Biol. Chem. 284, 19211–19219 (2009).
Jakulj, L. et al. Transintestinal cholesterol transport is active in mice and humans and controls ezetimibe-induced fecal neutral sterol excretion. Cell Metab. 24, 783–794 (2016).
Vrins, C. L. J. et al. Peroxisome proliferator-activated receptor delta activation leads to increased transintestinal cholesterol efflux. J. Lipid Res. 50, 2046–2054 (2009).
de Boer, J. F. et al. Intestinal farnesoid X receptor controls transintestinal cholesterol excretion in mice. Gastroenterology 152, 1126.e6–1138.e6 (2017).
Grefhorst, A. et al. Pharmacological LXR activation reduces presence of SR-B1 in liver membranes contributing to LXR-mediated induction of HDL-cholesterol. Atherosclerosis 222, 382–389 (2012).
de Boer, J. F., Kuipers, F. & Groen, A. K. Cholesterol transport revisited: a new turbo mechanism to drive cholesterol excretion. Trends Endocrinol. Metab. 29, 123–133 (2018).
Terunuma, S., Kumata, N. & Osada, K. Ezetimibe impairs uptake of dietary cholesterol oxidation products and reduces alterations in hepatic cholesterol metabolism and antioxidant function in rats. Lipids 48, 587–595 (2013).
van de Peppel, I. P. et al. Efficient reabsorption of transintestinally excreted cholesterol is a strong determinant for cholesterol disposal in mice. J. Lipid Res. 60, 1562–1572 (2019).
Hafkamp, A. M. et al. Novel kinetic insights into treatment of unconjugated hyperbilirubinemia: phototherapy and orlistat treatment in Gunn rats. Pediatr. Res. 59, 506–512 (2006).
Hafkamp, A. M., Havinga, R., Sinaasappel, M. & Verkade, H. J. Effective oral treatment of unconjugated hyperbilirubinemia in Gunn rats. Hepatology 41, 526–534 (2005).
Cuperus, F. J. C., Iemhoff, A. A. & Verkade, H. J. Combined treatment strategies for unconjugated hyperbilirubinemia in Gunn rats. Pediatr. Res 70, 560–565 (2011).
van der Velde, A. E. et al. Regulation of direct transintestinal cholesterol excretion in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 295, G203–G208 (2008).
Bulmer, A. C., Verkade, H. J. & Wagner, K. H. Bilirubin and beyond: a review of lipid status in Gilbert’s syndrome and its relevance to cardiovascular disease protection. Prog. Lipid Res. 52, 193–205 (2013).
Ronda, O. A. H. O., van Dijk, T. H., Verkade, H. J. & Groen, A. K. Measurement of intestinal and peripheral cholesterol fluxes by a dual-tracer balance method. Curr. Protoc. Mouse Biol. 6, 408–434 (2016).
Bligh, E. G. & Dyer, W. J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 (1959).
Heuman, D. M. Quantitative estimation of the hydrophilic-hydrophobic balance of mixed bile salt solutions. J. Lipid Res. 30, 719–730 (1989).
Xu, Y. et al. Farnesoid X receptor activation increases reverse cholesterol transport by modulating bile acid composition and cholesterol absorption in mice. Hepatology 64, 1072–1085 (2016).
Hambruch, E. et al. Synthetic farnesoid X receptor agonists induce high-density lipoprotein-mediated transhepatic cholesterol efflux in mice and monkeys and prevent atherosclerosis in cholesteryl ester transfer protein transgenic low-density lipoprotein receptor. J. Pharmacol. Exp. Ther. 343, 556–567 (2012).
Sugizaki, T. et al. The Niemann-Pick C1 Like 1 (NPC1L1) inhibitor ezetimibe improves metabolic disease via decreased liver X receptor (LXR) activity in liver of obese male mice. Endocrinology 155, 2810–2819 (2014).
Shih, A. W. Y., McFarlane, A. & Verhovsek, M. Haptoglobin testing in hemolysis: measurement and interpretation. Am. J. Hematol. 89, 443–447 (2014).
Nakano, T. et al. Ezetimibe promotes brush border membrane-to-lumen cholesterol efflux in the small intestine. PLoS ONE 11, e0152207 (2016).
Van Heek, M. et al. Comparison of the activity and disposition of the novel cholesterol absorption inhibitor, SCH58235, and its glucuronide, SCH60663. Br. J. Pharmacol. 129, 1748–1754 (2000).
Ghosal, A. et al. Identification of human UDP-glucuronosyltransferase enzyme(s) responsible for the glucuronidation of ezetimibe (Zetia). Drug Metab. Dispos. 32, 314–320 (2004).
Calpe-Berdiel, L. et al. Liver X receptor-mediated activation of reverse cholesterol transport from macrophages to feces in vivo requires ABCG5/G8. J. Lipid Res. 49, 1904–1911 (2008).
Oosterveer, M. H., Grefhorst, A., Groen, A. K. & Kuipers, F. The liver X receptor: control of cellular lipid homeostasis and beyond. Prog. Lipid Res. 49, 343–352 (2010).
Jonker, J. W., Liddle, C. & Downes, M. FXR and PXR: potential therapeutic targets in cholestasis. J. Steroid Biochem. Mol. Biol. 130, 147–158 (2012).
Van Der Veere, C. N. et al. Influence of dietary calcium phosphate on the disposition of bilirubin in rats with unconjugated hyperbilirubinemia. Hepatology 24, 620–626 (1996).
Duval, C. et al. Niemann–Pick C1 like 1 gene expression is down-regulated by LXR activators in the intestine. Biochem. Biophys. Res. Commun. 340, 1259–1263 (2006).
Kawase, A., Araki, Y., Ueda, Y., Nakazaki, S. & Iwaki, M. Impact of a high-cholesterol diet on expression levels of Niemann-Pick C1-like 1 and intestinal transporters in rats and mice. Eur. J. Drug Metab. Pharmacokinet. 41, 457–463 (2016).
Dimeski, G., Mollee, P. & Carter, A. Increased lipid concentration is associated with increased hemolysis. Clin. Chem. 51, 2425 (2005).
London, I. M., West, R., Shemin, D. & Rittenberg, D. On the origin of bile pigment in normal man. J. Biol. Chem. 184, 351–358 (1950).
Acknowledgements
The authors gratefully acknowledge the excellent technical contributions to this study by Rick Havinga, Renze Boverhof, Martijn Koehorst, and Anouk Bos. We thank Folkert Kuipers for helpful discussions and advice. This study was funded by a grant from De Cock Hadders stichting. L.V. received a grant from the Czech Ministry of Health (RVO-VFN64165).
Author information
Authors and Affiliations
Contributions
M.B. designed and performed the experiments, analyzed and interpreted data, and wrote the manuscript. I.P.v.d.P. interpreted data and wrote the manuscript. A.D. and N.C. analyzed data and reviewed the manuscript. L.V. interpreted data and reviewed the manuscript. J.W.J. and H.J.V. designed the experiments, interpreted data, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Blankestijn, M., van de Peppel, I.P., Dvorak, A. et al. Induction of fecal cholesterol excretion is not effective for the treatment of hyperbilirubinemia in Gunn rats. Pediatr Res 89, 510–517 (2021). https://doi.org/10.1038/s41390-020-0926-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-0926-2
This article is cited by
-
The TICE Pathway: Mechanisms and Potential Clinical Applications
Current Atherosclerosis Reports (2023)