ABSTRACT
Background
Inflammation is strongly associated with premature birth and neonatal morbidities. Increases in infant haptoglobin, haptoglobin-related protein (Hp&HpRP), and interleukin-6 (IL-6) levels are indicators of intra-amniotic inflammation (IAI) and have been linked to poor neonatal outcomes. Inflammation causes epigenetic changes, specifically suppression of miR-29 expression. The current study sought to determine whether miR-29b levels in cord blood or neonatal venous blood are associated with IAI, identified by elevated IL-6 and Hp, and subsequent clinical morbidities in the infant.
Methods
We tested 92 cord blood samples from premature newborns and 18 venous blood samples at 36 weeks corrected gestational age. MiR-29b, Hp&HpRP, and IL-6 were measured by polymerase chain reaction and enzyme-linked immunosorbent assay, respectively.
Results
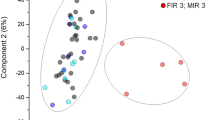
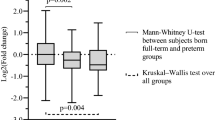
Decreased levels of miR-29b were observed in infants exposed to IAI with elevated Hp&HpRP and IL-6 levels and in infants delivered by spontaneous preterm birth. Lower miR-29 levels were also observed in women diagnosed with histological chorioamnionitis or funisitis and in infants with cerebral palsy. Higher levels of miR-29 were measured in infants small for gestational age and in venous samples from older infants.
Conclusions
MiR-29 may be an additional biomarker of IAI and a potential therapeutic target for treating poor newborn outcomes resulting from antenatal exposure to IAI.
Impact
-
Decreases in miR-29b are associated with intrauterine inflammation.
-
Hp&HpRP increases are associated with decreased miR-29b.
-
MiR-29b may be an additional biomarker for neonatal outcomes and a potential therapeutic target for intrauterine inflammation.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Preterm Birth. http:who.int/news-room/fact-sheets/detail/preterm-birth. (2018).
Premature Birth Report Cards. www.marchofdimes.org/mission/reportcard.aspx. (2018).
Glass, H. C. et al. Outcomes for extremely premature infants. Anesth. Analg. 120, 1337–1351 (2015).
Durrani-Kolarik, S. et al. miR-29b supplementation decreases expression of matrix proteins and improves alveolarization in mice exposed to maternal inflammation and neonatal hyperoxia. Am. J. Physiol. Lung Cell. Mol. Physiol. 313, L339–L349 (2017).
Beermann, J., Piccoli, M. T., Viereck, J. & Thum, T. Non-coding RNAs in development and disease: background, mechanisms, and therapeutic approaches. Physiol. Rev. 96, 1297–1325 (2016).
Stolzenburg, L. R. & Harris, A. The role of microRNAs in chronic respiratory disease: recent insights. Biol. Chem. 399, 219–234 (2018).
Quinlan, S., Kenny, A., Medina, M., Engel, T. & Jimenez-Mateos, E. M. MicroRNAs in neurodegenerative diseases. Int. Rev. Cell. Mol. Biol. 334, 309–343 (2017).
Wojciechowska, A., Braniewska, A. & Kozar-Kaminska, K. MicroRNA in cardiovascular biology and disease. Adv. Clin. Exp. Med. 26, 865–874 (2017).
Tang, Y. et al. The effect of H19-miR-29b interaction on bleomycin-induced mouse model of idiopathic pulmonary fibrosis. Biochem. Biophys. Res. Commun. 479, 417–423 (2016).
Yamada, Y. et al. Novel form of miR-29b suppresses bleomycin-induced pulmonary fibrosis. PLoS ONE 12, e0171957 (2017).
Tang, K., Zhao, J., Xie, J. & Wang, J. Decreased miR-29b expression is associated with airway inflammation in chronic obstructive pulmonary disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 316, L621–L629 (2019).
Velten, M. et al. Prenatal inflammation exacerbates hyperoxia-induced functional and structural changes in adult mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 303, R279–R290 (2012).
Velten, M., Heyob, K. M., Rogers, L. K. & Welty, S. E. Deficits in lung alveolarization and function after systemic maternal inflammation and neonatal hyperoxia exposure. J. Appl Physiol. (1985) 108, 1347–1356 (2010).
Cushing, L. et al. Disruption of miR-29 leads to aberrant differentiation of smooth muscle cells selectively associated with distal lung vasculature. PLoS Genet. 11, e1005238 (2015).
Montgomery, R. L. et al. MicroRNA mimicry blocks pulmonary fibrosis. EMBO Mol. Med. 6, 1347–1356 (2014).
Ng, P. C. et al. Early prediction of sepsis-induced disseminated intravascular coagulation with interleukin-10, interleukin-6, and RANTES in preterm infants. Clin. Chem. 52, 1181–1189 (2006).
Romagnoli, C. et al. Plasma levels of interleukin-6 and interleukin-10 in preterm neonates evaluated for sepsis. Eur. J. Pediatr. 160, 345–350 (2001).
Yoon, B. H. et al. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am. J. Obstet. Gynecol. 174, 1433–1440 (1996).
Goepfert, A. R. et al. Umbilical cord plasma interleukin-6 concentrations in preterm infants and risk of neonatal morbidity. Am. J. Obstet. Gynecol. 191, 1375–1381 (2004).
Rausen, A. R., Gerald, P. S. & Diamond, L. K. Haptoglobin patterns in cord blood serums. Nature 191, 717 (1961).
Kanakoudi, F. et al. Serum concentrations of 10 acute-phase proteins in healthy term and preterm infants from birth to age 6 months. Clin. Chem. 41, 605–608 (1995).
Buhimschi, C. S., Eunice Kennedy Shriver National Institute of Child H, Human Development Maternal-Fetal Medicine Units N. et al. Cord blood haptoglobin, cerebral palsy and death in infants of women at risk for preterm birth: a secondary analysis of a randomised controlled trial. EClinicalMedicine 9, 11–18 (2019).
Tseng, C. F., Lin, C. C., Huang, H. Y., Liu, H. C. & Mao, S. J. Antioxidant role of human haptoglobin. Proteomics 4, 2221–2228 (2004).
Buhimschi, C. S. et al. Proteomics mapping of cord blood identifies haptoglobin “switch-on” pattern as biomarker of early-onset neonatal sepsis in preterm newborns. PLoS ONE 6, e26111 (2011).
McCarthy, M. E. et al. Identification of haptoglobin switch-on status in archived placental specimens indicates antenatal exposure to inflammation and potential participation of the fetus in triggering preterm birth. Placenta 62, 50–57 (2018).
Barnard, G. A. Significance tests for 2 X 2 tables. Biometrika 34, 123–138 (1947).
Rabbee, N., Coull, B. A., Mehta, C., Patel, N. & Senchaudhuri, P. Power and sample size for ordered categorical data. Stat. Methods Med. Res. 12, 73–84 (2003).
Kruskal WHaW, W. A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 47, 583–621 (1952).
Committee on Obstetric P. Committee opinion no. 712: intrapartum management of intraamniotic infection. Obstet. Gynecol. 130, e95–e101 (2017).
Jobe, A. H. & Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 163, 1723–1729 (2001).
Luu, T. M., Rehman Mian, M. O. & Nuyt, A. M. Long-term impact of preterm birth: neurodevelopmental and physical health outcomes. Clin. Perinatol. 44, 305–314 (2017).
Patel, R. M. Short- and long-term outcomes for extremely preterm infants. Am. J. Perinatol. 33, 318–328 (2016).
Nuyt, A. M., Lavoie, J. C., Mohamed, I., Paquette, K. & Luu, T. M. Adult consequences of extremely preterm birth: cardiovascular and metabolic diseases risk factors, mechanisms, and prevention avenues. Clin. Perinatol. 44, 315–332 (2017).
Shi, W., Bellusci, S. & Warburton, D. Lung development and adult lung diseases. Chest 132, 651–656 (2007).
Wong, P. M. et al. Emphysema in young adult survivors of moderate-to-severe bronchopulmonary dysplasia. Eur. Respir. J. 32, 321–328 (2008).
Bhandari, V. et al. Cord blood erythropoietin and interleukin-6 for prediction of intraventricular hemorrhage in the preterm neonate. J. Matern. Fetal Neonatal Med. 24, 673–679 (2011).
Buhimschi, C. S. et al. Fetal inflammatory response in women with proteomic biomarkers characteristic of intra-amniotic inflammation and preterm birth. BJOG 116, 257–267 (2009).
Buhimschi, C. S. et al. Proteomic biomarkers of intra-amniotic inflammation: relationship with funisitis and early-onset sepsis in the premature neonate. Pediatr. Res. 61, 318–324 (2007).
Nayeri, U. A., Buhimschi, C. S., Zhao, G., Buhimschi, I. A. & Bhandari, V. Components of the antepartum, intrapartum, and postpartum exposome impact on distinct short-term adverse neonatal outcomes of premature infants: a prospective cohort study. PLoS ONE 13, e0207298 (2018).
Ment, L. R. et al. Intraventricular hemorrhage in the preterm neonate: timing and cerebral blood flow changes. J. Pediatr. 104, 419–425 (1984).
Guo, D. et al. Alteration in abundance and compartmentalization of inflammation-related miRNAs in plasma after intracerebral hemorrhage. Stroke 44, 1739–1742 (2013).
Samanta, S. et al. MicroRNA: a new therapeutic strategy for cardiovascular diseases. Trends Cardiovasc. Med. 26, 407–419 (2016).
Hayes, J., Peruzzi, P. P. & Lawler, S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol. Med. 20, 460–469 (2014).
Acknowledgements
We acknowledge the Ohio Perinatal Research Network Perinatal Research Repository for providing biospecimens and data for the project. These studies were support by NIH HD0880833.
Author information
Authors and Affiliations
Contributions
L.R.P. interpreted data and wrote the manuscript; S.V. performed biochemical analyses; C.W.B. performed statistical analyses of data; I.A.B. assisted in conceiving the idea, provided cord blood samples, and edited the manuscript; C.S.B. assisted in conceiving the idea, and edited the manuscript; L.K.R. conceived the idea, oversaw all analyses, and edited final draft of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent
Informed consent was obtained from the mother of the infants included in this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Pavlek, L.R., Vudatala, S., Bartlett, C.W. et al. MiR-29b is associated with perinatal inflammation in extremely preterm infants. Pediatr Res 89, 889–893 (2021). https://doi.org/10.1038/s41390-020-0943-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-0943-1