Abstract
Background
To evaluate the apoptosis inhibitor of macrophage (AIM) deposition patterns on the kidneys of children with IgA nephropathy (IgAN) and Henoch–Schönlein purpura nephritis (HSPN) and to investigate the clinical usefulness of serum and/or urinary AIM levels as biomarkers for the disease activity.
Methods
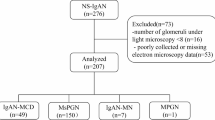
Immunohistochemical study was performed in the kidneys of 37 patients with IgAN and 10 patients with HSPN. Serum and urinary AIM levels in the patients and 20 healthy controls (HCs) were quantified by enzyme-linked immunosorbent assay. The results were compared with clinical features.
Results
In patients with IgAN and HSPN, AIM expression was observed in various areas, including the glomerular mesangial and capillary areas, the proximal and distal tubular epithelial cells, and on infiltrating macrophages in the glomeruli and interstitial areas. Serum and urinary AIM levels were significantly elevated in these patients compared with the HCs. Urinary AIM levels were positively correlated with the histological severity and degree of proteinuria and hematuria as well as urinary β2 microglobulin and urinary N-acetyl-β-D-glucosaminidase levels.
Conclusions
AIM plays an important role in the pathogenesis of IgAN and HSPN. Urinary AIM levels can potentially reflect active renal inflammation in these diseases and may represent a useful biomarker for disease activity.
Impact
-
Urinary AIM levels may represent a useful biomarker for disease activity of IgAN and HSPN.
-
AIM expression was observed in the glomeruli, tubular epithelial cells, and infiltrating macrophages in glomeruli and interstitial area.
-
U-AIM/Cr were significantly correlated not only with proteinuria, hematuria, and u-β2MG and u-NAG levels but also with the activity index of histological findings in kidney biopsy specimens.
-
Our results can emphasize the important role of AIM in the pathogenesis of IgAN and HSPN.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Miyazaki, T., Hirokami, Y., Matsuhashi, N., Takatsuka, H. & Naito, M. Increased susceptibility of thymocytes to apoptosis in mice lacking AIM, a novel murine macrophage-derived soluble factor belonging to the scavenger receptor cysteine-rich domain superfamily. J. Exp. Med. 189, 413–422 (1999).
Hamada, M. et al. MafB promotes atherosclerosis by inhibiting foam-cell apoptosis. Nat. Commun. 5, 3147 (2014).
Miyazaki, T., Kurokawa, J. & Arai, S. AIMing at metabolic syndrome. Towards the development of novel therapies for metabolic disease via apoptosis inhibitor of macrophage (AIM). Circ. J. 75, 2522–2531 (2011).
Arai, S. & Miyazaki, T. Impacts of the apoptosis inhibitor of macrophage (AIM) on obesity-associated inflammatory diseases. Semin. Immunopathol. 36, 3–12 (2014).
Mera, K. et al. Serum levels of apoptosis inhibitor of macrophage are associated with hepatic fibrosis in patients with chronic hepatitis C. BMC Gastroenterol. 14, 27 (2014).
Kojima, J. et al. Apoptosis inhibitor of macrophage (AIM) expression in alveolar macrophages in COPD. Respir. Res. 14, 30 (2013).
Haruta, I. et al. Apoptosis inhibitor expressed by macrophages tempers autoimmune colitis and the risk of colitis-based carcinogenesis in TCRalpha−/− mice. J. Clin. Immunol. 27, 549–556 (2007).
Balakrishnan, L. et al. Differential proteomic analysis of synovial fluid from rheumatoid arthritis and osteoarthritis patients. Clin. Proteomics 11, 1 (2014).
Lai, X., Xiang, Y., Zou, L., Li, Y. & Zhang, L. Elevation of serum CD5L concentration is correlated with disease activity in patients with systemic lupus erythematosus. Int. Immunopharmacol. 63, 311–316 (2018).
Yusa, S., Ohnishi, S., Onodera, T. & Miyazaki, T. AIM, a murine apoptosis inhibitory factor, induces strong and sustained growth inhibition of B lymphocytes in combination with TGF-beta1. Eur. J. Immunol. 29, 1086–1093 (1999).
Wang, C. et al. CD5L/AIM regulates lipid biosynthesis and restrains Th17 cell pathogenicity. Cell 163, 1413–1427 (2015).
Arai, S. et al. Apoptosis inhibitor of macrophage protein enhances intraluminal debris clearance and ameliorates acute kidney injury in mice. Nat. Med. 22, 183–193 (2016).
Andreoli, S. P., Yum, M. N. & Bergstein, J. M. IgA nephropathy in children: significance of glomerular basement membrane deposition of IgA. Am. J. Nephrol. 6, 28–33 (1986).
Morimoto, K. et al. Cytoprotective role of heme oxygenase (HO)-1 in human kidney with various renal diseases. Kidney Int. 60, 1858–1866 (2001).
Shimizu, M. et al. Glomerular proteinuria induces heme oxygenase-1 gene expression within renal epithelial cells. Pediatr. Res. 58, 666–671 (2005).
Striker, G. E., Mannik, M. & Tung, M. Y. Role of marrow-derived monocytes and mesangial cells in removal of immune complexes from renal glomeruli. J. Exp. Med. 149, 127–136 (1979).
Nathan, C. F., Murray, H. W. & Cohn, Z. A. The macrophage as an effector cell. N. Engl. J. Med. 303, 622–626 (1980).
Ricardo, S. D., van Goor, H. & Eddy, A. A. Macrophage diversity in renal injury and repair. J. Clin. Invest. 118, 3522–3530 (2008).
Lange-Sperandio, B., Fulda, S., Vandewalle, A. & Chevalier, R. L. Macrophages induce apoptosis in proximal tubule cells. Pediatr. Nephrol. 18, 335–341 (2003).
Oshima, M. et al. Association of apoptosis inhibitor of macrophage (AIM) expression with urinary protein and kidney dysfunction. Clin. Exp. Nephrol. 21, 35–42 (2017).
Author information
Authors and Affiliations
Contributions
H.I., M.S., S.K., N.I., M.M., Y.T., and K.O. were involved in the conception, design of the study, and the acquisition of data. H.I. and M.S. were involved in analysis, interpretation of data, and drafting of the manuscript. A.Y. and T.W. revised it critically for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Irabu, H., Shimizu, M., Kaneko, S. et al. Apoptosis inhibitor of macrophage as a biomarker for disease activity in Japanese children with IgA nephropathy and Henoch–Schönlein purpura nephritis. Pediatr Res 89, 667–672 (2021). https://doi.org/10.1038/s41390-020-0951-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-0951-1
This article is cited by
-
Telitacicept for childhood HSPN: A prospective self-controlled study
Clinical Rheumatology (2026)
-
The association between fractalkine/CX3CR1 axis with IgA vasculitis and nephritis
Pediatric Research (2025)
-
Administration of an antibody against apoptosis inhibitor of macrophage prevents aortic aneurysm progression in mice
Scientific Reports (2024)
-
Glomerular expression and urinary excretion of fatty acid-binding protein 4 in IgA nephropathy
Journal of Nephrology (2023)
-
IgA glycosylation and immune complex formation in IgAN
Seminars in Immunopathology (2021)