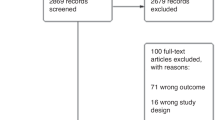
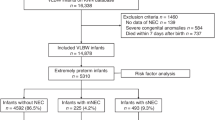
Abstract
Background
Infants with advanced necrotizing enterocolitis (NEC) often need surgical resection of necrotic bowel. We hypothesized that incomplete resection of NEC lesions, signified by the detection of necrotic patches in margins of resected bowel loops, results in inferior clinical outcomes.
Methods
We reviewed the medical records of infants with surgical NEC in the past 15 years for demographic, clinical, and histopathological data. We also developed statistical models to predict mortality and hospital stay.
Results
Ninety infants with surgical NEC had a mean (±standard error) gestational age of 27.3 ± 0.4 weeks, birth weight 1008 ± 48 g, NEC onset at 25.2 ± 2.4 days, and resected bowel length of 29.2 ± 3.2 cm. Seventeen (18.9%) infants who had complete resection of the necrosed bowel had fewer (4; 23.5%) deaths and shorter lengths of hospital stay. In contrast, a group of 73 infants with some necrosis within the margins of resected bowel had significantly more (34; 46.6%) deaths and longer hospital stay. The combination of clinical and histopathological data gave better regression models for mortality and hospital stay.
Conclusion
In surgical NEC, incomplete resection of necrotic bowel increased mortality and the duration of hospitalization. Regression models combining clinical and histopathological data were more accurate for mortality and the length of hospital stay.
Impact
-
In infants with surgical NEC, complete resection of necrotic bowel reduced mortality and hospital stay.
-
Regression models combining clinical and histopathological information were superior at predicting mortality and hospital stay than simpler models focusing on either of these two sets of data alone.
-
Prediction of mortality improved with the combination of antenatal steroids, chorioamnionitis, and duration of post-operative ileus, with severity of inflammation and hemorrhages in resected intestine.
-
Length of hospital stay was shorter in infants with higher gestational ages, but longer in those with greater depth of necrosis or needing prolonged parenteral nutrition or supervised feedings.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Neu, J. & Walker, W. A. Necrotizing enterocolitis. N. Engl. J. Med. 364, 255–264 (2011).
Ho, T. B. T. et al. Dichotomous development of the gut microbiome in preterm infants. Microbiome 6, 157 (2018).
Ho, T. B. T. et al. Enteric dysbiosis and fecal calprotectin expression in premature infants. Pediatr. Res. 85, 361–368 (2019).
Fundora, J. B., Guha P., Shores D. R., Pammi M. & Maheshwari A. Intestinal dysbiosis and necrotizing enterocolitis: assessment for causality using Bradford Hill criteria. Pediatr. Res. 87, 235–248 (2020).
MohanKumar, K. et al. Trinitrobenzene sulfonic acid-induced intestinal injury in neonatal mice activates transcriptional networks similar to those seen in human necrotizing enterocolitis. Pediatr. Res. 81, 99–112 (2016).
MohanKumar, K. et al. Gut mucosal injury in neonates is marked by macrophage infiltration in contrast to pleomorphic infiltrates in adult: evidence from an animal model. Am. J. Physiol. Gastrointest. Liver Physiol. 303, G93–G102 (2012).
Zhang, C. et al. Paneth cell ablation in the presence of Klebsiella pneumoniae induces necrotizing enterocolitis (NEC)-like injury in the small intestine of immature mice. Dis. Model. Mech. 5, 522–532 (2012).
MohanKumar, K. et al. A murine neonatal model of necrotizing enterocolitis caused by anemia and red blood cell transfusions. Nat. Commun. 10, 3494 (2019).
Garg, P. M., Hitt, M. M., Blackshear, C. & Maheshwari, A. Clinical determinants of postoperative outcomes in neonates with surgical necrotizing enterocolitis. J. Perinatol. (2020). in review.
Bowker, R. M., Yan, X. & De Plaen, I. G. Intestinal microcirculation and necrotizing enterocolitis: the vascular endothelial growth factor system. Semin. Fetal Neonatal Med. 23, 411–415 (2018).
Precioso, A. R. & Proenca, R. S. Necrotizing enterocolitis, pathogenesis and the protector effect of prenatal corticosteroids. Rev. Hosp. Clin. Fac. Med. Sao Paulo 57, 243–248 (2002).
Gellen, B. et al. Vascular changes play a role in the pathogenesis of necrotizing enterocolitis in asphyxiated newborn pigs. Pediatr. Surg. Int. 19, 380–384 (2003).
Eaton, S., Sebire, N., Thyoka, M. & Pierro, A. Histologic and immunohistochemical features associated with outcome in neonatal necrotizing enterocolitis. Eur. J. Pediatr. Surg. 24, 51–56 (2014).
Sheng, Q. et al. Short-term surgical outcomes of preterm infants with necrotizing enterocolitis: a single-center experience. Medicine (Baltim.) 95, e4379 (2016).
Wright, N. J. et al. The outcome of critically ill neonates undergoing laparotomy for necrotising enterocolitis in the neonatal intensive care unit: a 10-year review. J. Pediatr. Surg. 49, 1210–1214 (2014).
Li, X., Li, L., Wang, Y., Deng, C. & Guo, C. Postoperative characteristics of infants who developed necrotizing enterocolitis with different postnatal ages. Medicine (Baltim.) 96, e7774 (2017).
Hau, E. M. et al. Gastrointestinal sequelae after surgery for necrotising enterocolitis: a systematic review and meta-analysis. Arch. Dis. Child Fetal Neonatal Ed. 104, F265–F273 (2019).
Geng, Q., Wang, Y., Li, L. & Guo, C. Early postoperative outcomes of surgery for intestinal perforation in NEC based on intestinal location of disease. Medicine (Baltim.) 97, e12234 (2018).
Cole, C. R., Hansen, N. I., Higgins, R. D., Ziegler, T. R., Stoll, B. J. & Eunice Kennedy Shriver, N. N. R. N. Very low birth weight preterm infants with surgical short bowel syndrome: incidence, morbidity and mortality, and growth outcomes at 18 to 22 months. Pediatrics 122, e573–e582 (2008).
Ballance, W. A., Dahms, B. B., Shenker, N. & Kliegman, R. M. Pathology of neonatal necrotizing enterocolitis: a ten-year experience. J. Pediatr. 117, S6–S13 (1990).
Remon, J. I. et al. Depth of bacterial invasion in resected intestinal tissue predicts mortality in surgical necrotizing enterocolitis. J. Perinatol. 35, 755–762 (2015).
Yan, X. et al. Lack of VEGFR2 signaling causes maldevelopment of the intestinal microvasculature and facilitates necrotizing enterocolitis in neonatal mice. Am. J. Physiol. Gastrointest. Liver Physiol. 310, G716–G725 (2016).
Mutanen, A., Pierro, A. & Zani, A. Perioperative complications following surgery for necrotizing enterocolitis. Eur. J. Pediatr. Surg. 28, 148–151 (2018).
Pierro, A. & Hall, N. Surgical treatments of infants with necrotizing enterocolitis. Semin. Neonatol. 8, 223–232 (2003).
Blakely, M. L. et al. Postoperative outcomes of extremely low birth-weight infants with necrotizing enterocolitis or isolated intestinal perforation: a prospective cohort study by the NICHD Neonatal Research Network. Ann. Surg. 241, 984–989 (2005). discussion 989–994.
Jammeh, M. L. et al. Racial/ethnic differences in necrotizing enterocolitis incidence and outcomes in premature very low birth weight infants. J. Perinatol. 38, 1386–1390 (2018).
Stoll, B. J., Eunice Kennedy Shriver National Institute of Child H, Human Development Neonatal Research N. et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA 314, 1039–1051 (2015).
Bell, M. J., Feigin, R. D., Ternberg, J. L. & Brotherton, T. Evaluation of gastrointestinal microflora in necrotizing enterocolitis. J. Pediatr. 92, 589–592 (1978).
Gewolb, I. H., Schwalbe, R. S., Taciak, V. L., Harrison, T. S. & Panigrahi, P. Stool microflora in extremely low birthweight infants. Arch. Dis. Child Fetal Neonatal Ed. 80, F167–F173 (1999).
Smith, B. et al. Investigation of the early intestinal microflora in premature infants with/without necrotizing enterocolitis using two different methods. Pediatr. Res. 71, 115–120 (2012).
Watkins, D. J. & Besner, G. E. The role of the intestinal microcirculation in necrotizing enterocolitis. Semin. Pediatr. Surg. 22, 83–87 (2013).
Szabo, J. S., Mayfield, S. R., Oh, W. & Stonestreet, B. S. Postprandial gastrointestinal blood flow and oxygen consumption: effects of hypoxemia in neonatal piglets. Pediatr. Res. 21, 93–98 (1987).
Nowicki, P. T. et al. Intestinal O2 consumption in necrotizing enterocolitis: role of nitric oxide. Pediatr. Res. 59, 500–505 (2006).
Nowicki, P. T., Stonestreet, B. S., Hansen, N. B., Yao, A. C. & Oh, W. Gastrointestinal blood flow and oxygen consumption in awake newborn piglets: effect of feeding. Am. J. Physiol. 245, G697–G702 (1983).
Fernandez, R. et al. Identification of biomarkers of necrosis in xenografts using imaging mass spectrometry. J. Am. Soc. Mass Spectrom. 27, 244–254 (2016).
Tata, A. et al. Rapid detection of necrosis in breast cancer with desorption electrospray ionization mass spectrometry. Sci. Rep. 6, 35374 (2016).
Andrews, W. W. et al. The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. Am. J. Obstet. Gynecol. 195, 803–808 (2006).
Dorling, J., Kempley, S. & Leaf, A. Feeding growth restricted preterm infants with abnormal antenatal Doppler results. Arch. Dis. Child Fetal Neonatal Ed. 90, F359–F363 (2005).
Venkatesh, K. K. et al. Association of chorioamnionitis and its duration with neonatal morbidity and mortality. J. Perinatol. 39, 673–682 (2019).
Peterslund, P. et al. Frequencies of immune cells in the human small bowel during normal gestation and in necrotizing enterocolitis. Fetal Pediatr. Pathol. 38, 153–166 (2019).
Acknowledgements
We thank Dr. Neelesh Tipnis for helping us in developing and reviewing the manuscript. This research was funded by National Institutes of Health awards HL124078 and HL133022 (to A.M.).
Author information
Authors and Affiliations
Contributions
P.M.G., A.M., and A.G.S. designed the study. P.M.G., A.B., M.M.H., A.K., C.B., and A.G.S. collected and analyzed the data. All the authors contributed to and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Garg, P.M., Bernieh, A., Hitt, M.M. et al. Incomplete resection of necrotic bowel may increase mortality in infants with necrotizing enterocolitis. Pediatr Res 89, 163–170 (2021). https://doi.org/10.1038/s41390-020-0975-6
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-0975-6
This article is cited by
-
Outcomes by disease onset, sex, and intervention in neonates with SIP and surgical NEC
Pediatric Research (2024)
-
Clinical utilization of intestinal pathology in the classification of NEC vs SIP cases and prognostication
Journal of Perinatology (2024)
-
Postoperative Outcomes, and Growth and Brain Injury Outcomes in Spontaneous Intestinal Perforation vs Surgical Necrotizing Enterocolitis in Preterm Infants
Indian Pediatrics (2023)
-
Brain injury in preterm infants with surgical necrotizing enterocolitis: clinical and bowel pathological correlates
Pediatric Research (2022)
-
Early Career Investigator: Biocommentary
Pediatric Research (2021)