Abstract
Background
Neuromonitoring at the bedside is the key to understand the pathophysiological mechanisms of brain injury associated with neonatal encephalopathy. The current practice is to monitor the forehead using a noninvasive cerebral oximetry—it remains unknown to what extent cerebral hemodynamics in other brain regions is different to the frontal region.
Method
A multichannel near-infrared spectroscopy (NIRS) system was used to monitor neonates (n = 14) with fetal acidosis and mild neonatal encephalopathy at four brain regions (the frontal, posterior, left temporal, and right temporal lobes). The data were compared to delineate the regional difference in (1) cerebral hemodynamics and (2) pressure autoregulation. For both analyses, wavelet transform coherence was applied.
Results
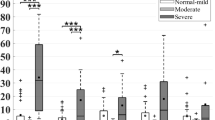
We observed frontal–posterior heterogeneity as indicated by significantly lower coherence between these two regions (p = 0.02). Furthermore, areas with regional magnetic resonance imaging (MRI)-detected lesions showed greater hemodynamic variations compared to non-affected areas (p = 0.03), while cerebral autoregulation was not affected and showed no difference.
Conclusion
Cerebral hemodynamics in mild neonatal encephalopathy is heterogeneous across different brain regions, while cerebral autoregulation remains intact. These findings indicate the robustness of the wavelet measure of cerebral autoregulation in this population, but need to be further investigated in the presence of severe injury.
Impact
-
This proof-of-concept study is the first to investigate the regional difference of cerebral hemodynamics and autoregulation in mild neonatal encephalopathy.
-
Study findings confirm that brain functions are complex in the developing neonatal brain and that cerebral hemodynamics are region specific in newborns with frontal–posterior heterogeneity among brain regions probed by multichannel NIRS.
-
Regional MRI lesions were associated with differences across NIRS regional channels among the affected side.
-
Cerebral autoregulation with multichannel NIRS is not affected by regional MRI abnormalities.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Chalak, L. F. Best practice guidelines on management of mild neonatal encephalopathy: Is it really mild? Early Hum Dev. 120, 74 (2018).
Greisen, G. Is near-infrared spectroscopy living up to its promises? Semin. Fetal Neonatal Med. 11, 498–502 (2006).
Tian, F. et al. Wavelet coherence analysis of dynamic cerebral autoregulation in neonatal hypoxic–ischemic encephalopathy. Neuroimaging Clin. 11, 124–132 (2016).
Sarnat, H. B. & Sarnat, M. S. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 33, 696–705 (1976).
Kopsas, L., Herman, P. & Eke, A. The modified Beer–Lambert law revisited. Philos. Med. Biol. 51, N91–N98 (2006).
Essenpreis, M. et al. Spectral dependence of temporal point spread functions in human tissues. Appl. Opt. 32, 418–425 (1993).
Tsuji, M. et al. Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics 106, 625–632 (2000).
Soul, J. S. et al. Fluctuating pressure-passivity is common in the cerebral circulation of sick premature infants. Pediatr Res. 61, 467–473 (2007).
Govindan, R. B. et al. Cerebral pressure passivity in newborns with encephalopathy undergoing therapeutic hypothermia. Front. Hum. Neurosci. 8, 266 (2014).
Grinsted, A., Moore, J. C. & Jevrejeva, S. Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Proc. Geophys. 11, 561–566 (2004).
Zhang, R. et al. Transfer function analysis of dynamic cerebral autoregulation in humans. Am. J. Physiol. 274, H233–H241 (1998).
Zhang, R. et al. Autonomic neural control of dynamic cerebral autoregulation in humans. Circulation 106, 1814–1820 (2002).
Chalak, L. F. et al. Neurodevelopmental outcomes after hypothermia therapy in the era of Bayley-III. J Perinatol 34, 629–633 (2014).
Rollins, N. et al. Predictive value of neonatal MRI showing no or minor degrees of brain injury after hypothermia. Pediatr. Neurol. 50, 447–451 (2014).
Lemmers, P. M. & van Bel, F. Left-to-right differences of regional cerebral oxygen saturation and oxygen extraction in preterm infants during the first days of life. Pediatr. Res. 65, 226–230 (2009).
Papademetriou, M. D. et al. Multichannel near infrared spectroscopy indicates regional variations in cerebral autoregulation in infants supported on extracorporeal membrane oxygenation. J. Biomed. Opt. 17, 067008 (2012).
Wagenaar, N. et al. Brain activity and cerebral oxygenation after perinatal arterial ischemic stroke. Stroke 50, 2668–2676 (2019).
Bar-Yosef, S., Sanders, E. G. & Grocott, H. P. Asymmetric cerebral near-infrared oximetric measurements during cardiac surgery. J. Cardiothorac. Vasc. Anesth. 17, 773–774 (2003).
Janelle, G. M. et al. Unilateral cerebral oxygen desaturation during emergent repair of a DeBakey type 1 aortic dissection: potential aversion of a major catastrophe. Anesthesiology 96, 1263–1265 (2002).
Sakamoto, T. et al. Cerebral ischemia caused by obstructed superior vena cava cannula is detected by near-infrared spectroscopy. J. Cardiothorac. Vasc. Anesth. 18, 293–303 (2004).
Chiron, C. et al. The right brain hemisphere is dominant in human infants. Brain 120, 1057–1065 (1997).
Volpe, J. J. Neurology of the newborn. Major Probl. Clin. Pediatr. 22, 1–648 (1981).
Richardson, B. S. et al. Webster regional blood flow change in the lamb during the perinatal period. Am. J. Obstet. Gynecol. 160, 919–925 (1989).
Kozberg, M. G. & Hillman, E. M. Neurovascular coupling develops alongside neural circuits in the postnatal brain. Neurogenesis (Austin) 3, e1244439 (2016).
Hendrikx, D. et al. Measurement of neurovascular coupling in neonates. Front. Physiol. 10, 65 (2019).
Pryds, O., Greisen, G. & Johansen, K. H. Indomethacin and cerebral blood flow in premature infants treated for patent ductus arteriosus. Eur. J. Pediatr. 147, 315–316 (1988).
Wong, F. Y. et al. Impaired autoregulation in preterm infants identified by using spatially resolved spectroscopy. Pediatrics 121, e604–e611 (2008).
Ouyang, M. et al. Heterogeneous increases of regional cerebral blood flow during preterm brain development: preliminary assessment with pseudo-continuous arterial spin labeled perfusion MRI. Neuroimaging 147, 233–242 (2017).
Acknowledgements
We thank all the families of subjects for their consent of participation. This study was supported by National Institutes of Health (NIH) Grant: 5R01NS102617-03 (to L.C.).
Author information
Authors and Affiliations
Contributions
F.T. performed the experiments, developed data analysis algorithms, analyzed the data, and prepared the manuscript. P.S. and S.K. assisted the subject recruitment, experimental preparation, and data acquisition. Y.D. participated in data acquisition and result interpretation. Y.L. performed statistical imputation and interpretation, H.L. and R.Z. discussed and interpreted the results, and revised the manuscript. L.C. initiated and supervised the study, interpreted the results, and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent to participate
This study was approved by IRB and consent was obtained.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tian, F., Sepulveda, P., Kota, S. et al. Regional heterogeneity of cerebral hemodynamics in mild neonatal encephalopathy measured with multichannel near-infrared spectroscopy. Pediatr Res 89, 882–888 (2021). https://doi.org/10.1038/s41390-020-0992-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-0992-5