Abstract
Background
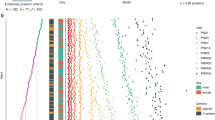
Premature birth entails an adverse cardiovascular risk profile, but the underlying mechanisms are insufficiently understood. Here, we employed an unbiased cardiovascular proteomics approach to profile former very preterm-born preschoolers.
Methods
This observational study investigated differences in plasma concentrations of 79 proteins, including putative cardiovascular biomarkers between very preterm- and term-born children on average 5.5 years old (53.1% male) using multiple-reaction monitoring mass spectrometry.
Results
Very preterm-born (n = 38; median gestational age 29.6 weeks) compared to term-born (n = 26; 40.2 weeks) children featured lower plasma concentrations of platelet factor 4 (PLF4; −61.6%, P < 0.0001), platelet basic protein (CXCL7; −57.8%, P < 0.0001), and hemoglobin subunit beta (−48.3%, P < 0.0001). Results remained virtually unchanged when adjusting for complete blood count parameters, including platelet count. Conversely, whole blood hemoglobin was higher (+7.62%, P < 0.0001) in preterm-born children.
Conclusions
Very preterm birth was associated with decreased markers of platelet activation among preschoolers. These findings are consistent with reduced platelet reactivity persisting from very preterm birth to a preschool age.
Impact
-
Former very preterm-born preschoolers featured reduced levels of platelet activation markers.
-
While lower platelet reactivity in very preterm-born compared to term-born infants in the first days of life was established, it was unknown when, if at all, reactivity normalizes. The current study suggests that platelet hyporeactivity due to very preterm birth persists at least up to a preschool age.
-
“Immaturity of the hemostatic system” may be a persistent sequel of preterm birth, but larger studies are needed to investigate its potential clinical implications.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bayman, E., Drake, A. J. & Piyasena, C. Prematurity and programming of cardiovascular disease risk: a future challenge for public health? Arch. Dis. Child. Fetal Neonatal Ed. 99, F510–F514 (2014).
Komazec, I. O. et al. Aortic elastic properties in preschool children born preterm. Arterioscler. Thromb. Vasc. Biol. 36, 2268–2274 (2016).
Stoll, B. J. et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA 314, 1039–1051 (2015).
Vohr, B. R., Allan, W., Katz, K. H., Schneider, K. C. & Ment, L. R. Early predictors of hypertension in prematurely born adolescents. Acta Paediatr. 99, 1812–1818 (2010).
Posod, A. et al. Former very preterm infants show an unfavorable cardiovascular risk profile at a preschool age. PLoS ONE 11, e0168162 (2016).
Stock, K. et al. The impact of being born preterm or small for gestational age on early vascular aging in adolescents. J. Pediatr. 201, 49–54 (2018).
Lewandowski, A. J. et al. Right ventricular systolic dysfunction in young adults born preterm. Circulation 128, 713–720 (2013).
Huckstep, O. J. et al. Physiological stress elicits impaired left ventricular function in preterm-born adults. J. Am. Coll. Cardiol. 71, 1347–1356 (2018).
Sola-Visner, M. Platelets in the neonatal period: developmental differences in platelet production, function, and hemostasis and the potential impact of therapies. ASH Educ. Program Book 2012, 506–511 (2012).
Markopoulou, P., Papanikolaou, E., Analytis, A., Zoumakis, E. & Siahanidou, T. Preterm birth as a risk factor for metabolic syndrome and cardiovascular disease in adult life: a systematic review and meta-analysis. J. Pediatr. 210, 69–80 (2019).
Keijzer-Veen, M. G. et al. Reduced renal length and volume 20 years after very preterm birth. Pediatr. Nephrol. Berl. Ger. 25, 499–507 (2010).
Crump, C., Sundquist, J., Winkleby, M. A. & Sundquist, K. Preterm birth and risk of chronic kidney disease from childhood into mid-adulthood: National Cohort Study. BMJ 365, l1346 (2019).
Brummelte, S. et al. Cortisol levels in former preterm children at school age are predicted by neonatal procedural pain-related stress. Psychoneuroendocrinology 51, 151–163 (2015).
Posod, A. et al. Former very preterm infants show alterations in thyroid function at a preschool age. BioMed. Res. Int. 2017, 3805370 (2017).
Fenton, T. R. & Kim, J. H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 13, 59 (2013).
Kromeyer-Hauschild, K. et al. Perzentile für den Body-mass-Index für das Kindes- und Jugendalter unter Heranziehung verschiedener deutscher Stichproben. Monatsschr. Kinderheilkd. 149, 807–818 (2001).
Posod, A. et al. Apolipoprotein profiles in very preterm and term‐born preschool children. J. Am. Heart Assoc. 8 (2019).
Eden, E., Navon, R., Steinfeld, I., Lipson, D. & Yakhini, Z. GOrilla: a tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinform. 10, 48 (2009).
Yun, S.-H., Sim, E.-H., Goh, R.-Y., Park, J.-I. & Han, J.-Y. Platelet activation: the mechanisms and potential biomarkers. BioMed. Res. Int. 2016, 9060143 (2016).
Kowalska, M. A., Rauova, L. & Poncz, M. Role of the platelet chemokine platelet factor 4 (PF4) in hemostasis and thrombosis. Thromb. Res. 125, 292–296 (2010).
DeLoughery, T. G. Coagulation abnormalities and cardiovascular disease. Curr. Opin. Lipidol. 10, 443–448 (1999).
Israels, S. J., Rand, M. L. & Michelson, A. D. Neonatal platelet function. Semin. Thromb. Hemost. 29, 363–372 (2003).
Sitaru, A. G. et al. Neonatal platelets from cord blood and peripheral blood. Platelets 16, 203–210 (2005).
Bednarek, F. J., Bean, S., Barnard, M. R., Frelinger, A. L. & Michelson, A. D. The platelet hyporeactivity of extremely low birth weight neonates is age-dependent. Thromb. Res. 124, 42–45 (2009).
Uçar, T., Gurman, C., Arsan, S. & Kemahli, S. Platelet aggregation in term and preterm newborns. Pediatr. Hematol. Oncol. 22, 139–145 (2005).
Hézard, N. et al. Unexpected persistence of platelet hyporeactivity beyond the neonatal period: a flow cytometric study in neonates, infants and older children. Thromb. Haemost. 90, 116–123 (2003).
Linder, N. et al. Deposition of whole blood platelets on extracellular matrix under flow conditions in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 86, 127F–130F (2002).
Jackson, S. P. The growing complexity of platelet aggregation. Blood 109, 5087–5095 (2007).
Flahault, A. et al. Duration of neonatal oxygen supplementation, erythropoiesis and blood pressure in young adults born preterm. Thorax 75, 494–502 (2020).
Vollsæter, M. et al. Adult respiratory outcomes of extreme preterm birth. A regional cohort study. Ann. Am. Thorac. Soc. 12, 313–322 (2015).
Olivieri, N. F. Fetal erythropoiesis and the diagnosis and treatment of hemoglobin disorders in the fetus and child. Semin. Perinatol. 21, 63–69 (1997).
Andrew, M., Paes, B. & Johnston, M. Development of the hemostatic system in the neonate and young infant. Am. J. Pediatr. Hematol. Oncol. 12, 95–104 (1990).
Del Vecchio, A., Latini, G., Henry, E. & Christensen, R. D. Template bleeding times of 240 neonates born at 24 to 41 weeks gestation. J. Perinatol. 28, 427–431 (2008).
Boudewijns, M. et al. Evaluation of platelet function on cord blood in 80 healthy term neonates using the Platelet Function Analyser (PFA-100); shorter in vitro bleeding times in neonates than adults. Eur. J. Pediatr. 162, 212–213 (2003).
Saxonhouse, M. A. et al. Closure times measured by the Platelet Function Analyzer PFA-100® are longer in neonatal blood compared to cord blood samples. Neonatology 97, 242–249 (2010).
Arepally, G. M. Heparin-induced thrombocytopenia. Blood 129, 2864–2872 (2017).
Warkentin, T. E. et al. Sera from patients with heparin-induced thrombocytopenia generate platelet-derived microparticles with procoagulant activity: an explanation for the thrombotic complications of heparin-induced thrombocytopenia. Blood 84, 3691–3699 (1994).
Amiral, J. & Vissac, A. M. Generation and pathogenicity of anti-platelet factor 4 antibodies: diagnostic implications. Clin. Appl. Thromb. Hemost. 5, S28–S31 (1999).
Poncz, M., Rauova, L. & Cines, D. B. The role of surface PF4: glycosaminoglycan complexes in the pathogenesis of heparin-induced thrombocytopenia (HIT). Pathophysiol. Haemost. Thromb. 35, 46–49 (2006).
Mattioli, A. V., Bonetti, L., Zennaro, M., Ambrosio, G. & Mattioli, G. Heparin/PF4 antibodies formation after heparin treatment: temporal aspects and long-term follow-up. Am. Heart J. 157, 589–595 (2009).
Acknowledgements
This study was supported by the Anniversary Fund of the Austrian National Bank (Project No. 14570) and the Austrian Heart Foundation, and by VASCage—Research Center on Vascular Ageing and Stroke (No. 868624). As a COMET center, VASCage is funded within the COMET program—Competence Centers for Excellent Technologies by the Austrian Ministry for Climate Action, Environment, Energy, Mobility, Innovation, and Technology, the Austrian Ministry for Digital and Economic Affairs, and the federal states Tyrol, Salzburg, and Vienna. Prof. Manuel Mayr is a British Heart Foundation (BHF) Chair Holder (CH/16/3/32406) with BHF program grant support (RG/16/14/32397). This work was supported by the National Institute of Health Research (NIHR) Biomedical Research Center based at Guy’s and St. Thomas’ National Health Service (NHS) Foundation Trust and King’s College London in partnership with King’s College Hospital.
Author information
Authors and Affiliations
Contributions
Designed the study: U.K.-K. and A.P. Obtained funding: U.K.-K. and S.K. Supervised the study: U.K.-K. Analyzed the data: R.P. Drafted the manuscript: R.P. Revised the manuscript for important intellectual content: A.P., X.Y., S.A.P., S.Z.-K., M.M., S.K., and U.K.-K. Performed proteomics measurements: X.Y. and S.A.P. Approved the final version of the manuscript submitted: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Statement of consent
All children included in this study consented to participation orally, and written informed consent was obtained from their legal guardians.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pechlaner, R., Posod, A., Yin, X. et al. Very preterm birth results in later lower platelet activation markers. Pediatr Res 89, 1278–1282 (2021). https://doi.org/10.1038/s41390-020-1070-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-1070-8
This article is cited by
-
Exposure to high levels of oxygen in neonatal rats induce a decrease in hemoglobin levels
Pediatric Research (2022)