Abstract
Background
Fetal hypoxia has been implicated in fetal growth restriction in congenital heart disease (CHD) and leads to stress erythropoiesis in utero. The objective is to assess erythropoiesis and its association with growth in newborns with CHD.
Methods
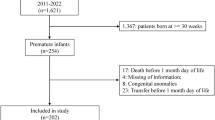
Fetuses with prenatally diagnosed CHD from 2013 to 2018 were retrospectively reviewed. Pregnancies with multiple gestation, genetic abnormalities, major extra-cardiac anomalies, and placental abruption were excluded. Complete blood count tests at birth were compared to published normative values. Spearman correlation assessed associations of red blood cell (RBC) indices with birth anthropometrics and prenatal Doppler measures.
Results
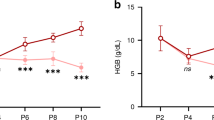
A total of 160 newborns were included. Median gestational age was 38.3 (37.3, 39.0) weeks. Infants ≥37 weeks gestation had lower hemoglobin (Hgb), hematocrit, and elevated nucleated RBC (nRBC), mean corpuscular volume, and mean corpuscular hemoglobin compared to reference. No differences in RBC indices were observed in infants <34 and 34–37 weeks gestation. There was no difference in Hgb and nRBC between CHD subgroups. Neither Hgb nor nRBC were associated with birth anthropometrics or Doppler patterns.
Conclusions
Term infants with CHD demonstrated multiple alterations in erythrocyte indices suggesting ineffective stress erythropoiesis in late gestation resulting in lower Hgb at birth. Altered erythropoiesis was not correlated to growth or Doppler patterns.
Impact
-
Newborns with congenital heart disease (CHD) born at term gestation demonstrated altered erythropoiesis.
-
Term newborns with CHD have decreased hemoglobin levels despite having red blood cell indices consistent with stress erythropoiesis, suggesting an incomplete compensatory response to in utero physiologic disturbances associated with CHD.
-
The etiology is unknown; however, it may be influenced by multiple risk factors during pregnancy in the maternal–fetal dyad.
-
Alterations in red blood cell indices were not associated with outcomes of fetal growth.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Alsoufi, B. et al. Low-weight infants are at increased mortality risk after palliative or corrective cardiac surgery. J. Thorac. Cardiovasc. Surg. 148, 2508.e1–2514.e1 (2014).
Best, K. E., Tennant, P. W. G. & Rankin, J. Survival, by birth weight and gestational age, in individuals with congenital heart disease: a population-based study. J. Am. Heart Assoc. 6, e005213 (2017).
Ades, A. M. et al. Morbidity and mortality after surgery for congenital cardiac disease in the infant born with low weight. Cardiol. Young 20, 8–17 (2010).
Malik, S. et al. Association between congenital heart defects and small for gestational age. Pediatrics 119, e976–e982 (2007).
Wallenstein, M. B. et al. Fetal congenital heart disease and intrauterine growth restriction: a retrospective cohort study. J. Matern. Fetal Neonatal Med. 25, 662–665 (2012).
Malhotra, A. et al. Neonatal morbidities of fetal growth restriction: pathophysiology and impact. Front. Endocrinol. 10, 55 (2019).
Krishna, U. & Bhalerao, S. Placental insufficiency and fetal growth restriction. J. Obstet. Gynaecol. India 61, 505–511 (2011).
Rychik, J. et al. Characterization of the placenta in the newborn with congenital heart disease: distinctions based on type of cardiac malformation. Pediatr. Cardiol. 39, 1165–1171 (2018).
Rosenthal, G. L. Patterns of prenatal growth among infants with cardiovascular malformations: possible fetal hemodynamic effects. Am. J. Epidemiol. 143, 505–513 (1996).
Rosenthal, G. L., Wilson, P. D., Permutt, T., Boughman, J. A. & Ferencz, C. Birth weight and cardiovascular malformations: a population-based study. The Baltimore-Washington Infant Study. Am. J. Epidemiol. 133, 1273–1281 (1991).
Puri, K. et al. Fetal somatic growth trajectory differs by type of congenital heart disease. Pediatr. Res. 83, 669–676 (2018).
Nicolaides, K. H., Economides, D. L. & Soothill, P. W. Blood gases, pH, and lactate in appropriate- and small-for-gestational-age fetuses. Am. J. Obstet. Gynecol. 161, 996–1001 (1989).
Parra-Saavedra, M. et al. Association of Doppler parameters with placental signs of underperfusion in late-onset small-for-gestational-age pregnancies. Ultrasound Obstet. Gynecol. 44, 330–337 (2014).
Wedegartner, U. et al. T2 and T2* measurements of fetal brain oxygenation during hypoxia with MRI at 3T: correlation with fetal arterial blood oxygen saturation. Eur. Radiol. 20, 121–127 (2010).
Nicolaides, K. H., Bilardo, C. M., Soothill, P. W. & Campbell, S. Absence of end diastolic frequencies in umbilical artery: a sign of fetal hypoxia and acidosis. BMJ 297, 1026–1027 (1988).
Siristatidis, C., Salamalekis, E., Kassanos, D., Loghis, C. & Creatsas, G. Evaluation of fetal intrapartum hypoxia by middle cerebral and umbilical artery Doppler velocimetry with simultaneous cardiotocography and pulse oximetry. Arch. Gynecol. Obstet. 270, 265–270 (2004).
Vyas, S., Nicolaides, K. H., Bower, S. & Campbell, S. Middle cerebral artery flow velocity waveforms in fetal hypoxaemia. Br. J. Obstet. Gynaecol. 97, 797–803 (1990).
Krampl, E. et al. Fetal Doppler velocimetry at high altitude. Ultrasound Obstet. Gynecol. 18, 329–334 (2001).
Sun, L. et al. Understanding fetal hemodynamics using cardiovascular magnetic resonance imaging. Fetal Diagn. Ther. 47, 354–362 (2020).
Teramo, K. A. & Widness, J. A. Increased fetal plasma and amniotic fluid erythropoietin concentrations: markers of intrauterine hypoxia. Neonatology 95, 105–116 (2009).
Hermansen, M. C. Nucleated red blood cells in the fetus and newborn. Arch. Dis. Child. Fetal Neonatal Ed. 84, F211–F215 (2001).
Neerhof, M. G. & Thaete, L. G. The fetal response to chronic placental insufficiency. Semin. Perinatol. 32, 201–205 (2008).
Bresnick, E. H. et al. Mechanisms of erythrocyte development and regeneration: implications for regenerative medicine and beyond. Development 145, dev151423 (2018).
Gidding, S. S. & Stockman, J. A. III Erythropoietin in cyanotic heart disease. Am. Heart J. 116, 128–132 (1988).
American College of Obstetricians and Gynecologists. ACOG Practice bulletin no. 134: fetal growth restriction. Obstet. Gynecol. 121, 1122–1133 (2013).
Perrone, S. et al. Nucleated red blood cell count in term and preterm newborns: reference values at birth. Arch. Dis. Child. Fetal Neonatal Ed. 90, F174–F175 (2005).
Gosling, R. G. et al. The quantitative analysis of occlusive peripheral arterial disease by a non-intrusive ultrasonic technique. Angiology 22, 52–55 (1971).
Jopling, J., Henry, E., Wiedmeier, S. E. & Christensen, R. D. Reference ranges for hematocrit and blood hemoglobin concentration during the neonatal period: data from a multihospital health care system. Pediatrics 123, e333–e337 (2009).
Christensen, R. D., Jopling, J., Henry, E. & Wiedmeier, S. E. The erythrocyte indices of neonates, defined using data from over 12,000 patients in a multihospital health care system. J. Perinatol. 28, 24–28 (2008).
Christensen, R. D., Yaish, H. M., Henry, E. & Bennett, S. T. Red blood cell distribution width: reference intervals for neonates. J. Matern. Fetal Neonatal Med. 28, 883–888 (2015).
Hanion-Lundberg, K. M., Kirby, R. S., Gandhi, S. & Broekhuizen, F. F. Nucleated red blood cells in cord blood of singleton term neonates. Am. J. Obstet. Gynecol. 176, 1149–1154 (1997).
Palis, J. & Segel, G. B. Developmental biology of erythropoiesis. Blood Rev. 12, 106–114 (1998).
Finne, P. H. & Halvorsen, S. Regulation of erythropoiesis in the fetus and newborn. Arch. Dis. Child. 47, 683–687 (1972).
Kuruvilla, D. J. et al. A method to evaluate fetal erythropoiesis from postnatal survival of fetal RBCs. AAPS J. 17, 1246–1254 (2015).
Thilaganathan, B. et al. Umbilical cord blood erythroblast count as an index of intrauterine hypoxia. Arch. Dis. Child. Fetal Neonatal Ed. 70, F192–F194 (1994).
Jones, H. N. et al. Hypoplastic left heart syndrome is associated with structural and vascular placental abnormalities and leptin dysregulation. Placenta 36, 1078–1086 (2015).
Steurer, M. A. et al. Impaired fetal environment and gestational age: what is driving mortality in neonates with critical congenital heart disease? J. Am. Heart Assoc. 8, e013194 (2019).
Evseenko, D. A. & Tsirel’nikov, N. I. Role of placenta in the regulation of fetal erythropoiesis. Bull. Exp. Biol. Med. 132, 1055–1057 (2001).
Tabbah, S. M. et al. Hepcidin, an iron regulatory hormone of innate immunity, is differentially expressed in premature fetuses with early-onset neonatal sepsis. Am. J. Perinatol. 35, 865–872 (2018).
Moore, L. G., Charles, S. M. & Julian, C. G. Humans at high altitude: hypoxia and fetal growth. Respir. Physiol. Neurobiol. 178, 181–190 (2011).
Miller, J., Turan, S. & Baschat, A. A. Fetal growth restriction. Semin. Perinatol. 32, 274–280 (2008).
Acharya, G., Wilsgaard, T., Berntsen, G. K., Maltau, J. M. & Kiserud, T. Reference ranges for serial measurements of umbilical artery Doppler indices in the second half of pregnancy. Am. J. Obstet. Gynecol. 192, 937–944 (2005).
Ebbing, C., Rasmussen, S. & Kiserud, T. Middle cerebral artery blood flow velocities and pulsatility index and the cerebroplacental pulsatility ratio: longitudinal reference ranges and terms for serial measurements. Ultrasound Obstet. Gynecol. 30, 287–296 (2007).
Yang, Z. W. et al. Comparison of blood counts in venous, fingertip and arterial blood and their measurement variation. Clin. Lab. Haematol. 23, 155–159 (2001).
Patel, A. J., Wesley, R., Leitman, S. F. & Bryant, B. J. Capillary versus venous haemoglobin determination in the assessment of healthy blood donors. Vox Sang. 104, 317–323 (2013).
Author information
Authors and Affiliations
Contributions
Each author has met authorship requirements. S.Y.T. conceptualized and designed the study, acquired data, performed data interpretation, and drafted and critically revised the manuscript. Z.G. and N.J.O. assisted with study design, carried out data analyses and interpretation, and critically revised the manuscript. T.A.K. and S.T. assisted with study design and critically revised the manuscript. R.K. collected data and reviewed the manuscript. J.F.C. conceptualized and designed the study, coordinated and supervised data collection and data analysis, performed data interpretation, and critically revised the manuscript. All authors approved the final version for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Patient consent
Patient consent was not required for this study. Approval was obtained from the Institutional Review Board at Cincinnati Children’s Hospital Medical Center with waiver of consent due to the retrospective nature of the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tseng, S.Y., Gao, Z., Kalfa, T.A. et al. Altered erythropoiesis in newborns with congenital heart disease. Pediatr Res 91, 606–611 (2022). https://doi.org/10.1038/s41390-021-01370-4
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-021-01370-4
This article is cited by
-
Nucleated Red Blood Cell Counts Differentiate Cardiac from Respiratory Causes of Cyanosis at Birth
Pediatric Cardiology (2024)